The Design and Implementation of a New Surveillance System for Venous Thromboembolism Using Combined Active and Passive Methods
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Intravenous Drug Use-Associated Infective Endocarditis in Pregnant Patients at a Hospital in West Virginia
Open Access Original Article DOI: 10.7759/cureus.17218 Intravenous Drug Use-Associated Infective Endocarditis in Pregnant Patients at a Hospital in West Virginia Deena Dahshan 1 , Mohamed Suliman 2 , Ebad U. Rahman 3 , Zachary Curtis 1 , Ellen Thompson 2 1. Internal Medicine, Marshall University Joan C. Edwards School of Medicine, Huntington, USA 2. Cardiology, Marshall University Joan C. Edwards School of Medicine, Huntington, USA 3. Internal Medicine, St. Mary's Medical Center, Huntington, USA Corresponding author: Deena Dahshan, [email protected] Abstract Introduction Due to high levels of intravenous drug use (IVDU) in West Virginia (WV), there are increasing numbers of hospitalizations for infective endocarditis (IE). More specifically, pregnant patients with IE are a uniquely challenging population, with complex management and a clinical course that further affects the health of the fetus, with high morbidity and mortality. Timely recognition and awareness of the most common bacterial causes will provide hospitals and clinicians with valuable information to manage future patients. Methods This retrospective study analyzed the clinical course of pregnant patients admitted with IE and IVDU history presenting at Cabell Huntington Hospital from 2013 to 2018. Inclusion criteria were women between 16 and 45 years of age confirmed to be pregnant by urine pregnancy test and ultrasonography with at least eight weeks gestation, with a first-time diagnosis of endocarditis and an identified history of IVDU. We excluded charts with pre-existing risk factors including a history of valvular disease, rheumatic heart disease, surgical valve repair or mechanical valve replacement, or a diagnosis of coagulopathies. The resulting charts were evaluated for isolated organisms, reported clinical course, and complications of the pregnancy. -

Diseases of the Digestive System (KOO-K93)
CHAPTER XI Diseases of the digestive system (KOO-K93) Diseases of oral cavity, salivary glands and jaws (KOO-K14) lijell Diseases of pulp and periapical tissues 1m Dentofacial anomalies [including malocclusion] Excludes: hemifacial atrophy or hypertrophy (Q67.4) K07 .0 Major anomalies of jaw size Hyperplasia, hypoplasia: • mandibular • maxillary Macrognathism (mandibular)(maxillary) Micrognathism (mandibular)( maxillary) Excludes: acromegaly (E22.0) Robin's syndrome (087.07) K07 .1 Anomalies of jaw-cranial base relationship Asymmetry of jaw Prognathism (mandibular)( maxillary) Retrognathism (mandibular)(maxillary) K07.2 Anomalies of dental arch relationship Cross bite (anterior)(posterior) Dis to-occlusion Mesio-occlusion Midline deviation of dental arch Openbite (anterior )(posterior) Overbite (excessive): • deep • horizontal • vertical Overjet Posterior lingual occlusion of mandibular teeth 289 ICO-N A K07.3 Anomalies of tooth position Crowding Diastema Displacement of tooth or teeth Rotation Spacing, abnormal Transposition Impacted or embedded teeth with abnormal position of such teeth or adjacent teeth K07.4 Malocclusion, unspecified K07.5 Dentofacial functional abnormalities Abnormal jaw closure Malocclusion due to: • abnormal swallowing • mouth breathing • tongue, lip or finger habits K07.6 Temporomandibular joint disorders Costen's complex or syndrome Derangement of temporomandibular joint Snapping jaw Temporomandibular joint-pain-dysfunction syndrome Excludes: current temporomandibular joint: • dislocation (S03.0) • strain (S03.4) K07.8 Other dentofacial anomalies K07.9 Dentofacial anomaly, unspecified 1m Stomatitis and related lesions K12.0 Recurrent oral aphthae Aphthous stomatitis (major)(minor) Bednar's aphthae Periadenitis mucosa necrotica recurrens Recurrent aphthous ulcer Stomatitis herpetiformis 290 DISEASES OF THE DIGESTIVE SYSTEM Diseases of oesophagus, stomach and duodenum (K20-K31) Ill Oesophagitis Abscess of oesophagus Oesophagitis: • NOS • chemical • peptic Use additional external cause code (Chapter XX), if desired, to identify cause. -

State Medicaid Manual
Page 1 of 37 Louisiana Medicaid Approved Pay and Chase Primary Prenatal Care Diagnosis Codes MCOs must use the "pay and chase" method of payment for prenatal services for individuals with other Health Insurance. The MCO must seek reimbursement from the third party within 60 days after the end of the month in which payment is made. Primary prenatal diagnoses which do not require primary health insurance claim filing by most providers are confined to those listed below. Hospitals are not included and must continue to file claims with the primary health insurance carriers. ICD-9-CM to ICD-10 crosswalk for Prenatal Diagnosis Codes ICD-9-CM Description Code V22.0 Supervision of normal pregnancy V22.1 V23 Supervision of high risk pregnancy V28 Antenatal screening 640-648 Complications related to pregnancy 651-658 Other conditions requiring care in 671 pregnancy 673 675-676 ICD-10-CM Diagnosis Codes – for Prenatal Services upon Implementation of ICD-10 ICD-9-CM code V22.0 maps to the following ICD-10-CM codes Z3400 Encounter for supervision of normal first pregnancy, unspecified trimester Z3403 Encounter for supervision of normal first pregnancy, third trimester Z3401 Encounter for supervision of normal first pregnancy, first trimester Z3402 Encounter for supervision of normal first pregnancy, second trimester ICD-9-CM code V22. -

Supplementary Data
ESC Guidelines 1 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): supplementary data The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC) Authors/Task Force Members: Stavros V. Konstantinides* (Chairperson) (Germany/Greece), Guy Meyer* (Co-Chairperson) (France), Cecilia Becattini (Italy), Héctor Bueno (Spain), Geert-Jan Geersing (Netherlands), Veli-Pekka Harjola (Finland), Menno V. Huisman (Netherlands), Marc Humbert1 (France), Catriona Sian Jennings (United Kingdom), David Jiménez (Spain), Nils Kucher (Switzerland), Irene Marthe Lang (Austria), Mareike Lankeit (Germany), Roberto Lorusso (Netherlands), Lucia Mazzolai (Switzerland), Nicolas Meneveau (France), Fionnuala Ní Áinle (Ireland), Paolo Prandoni (Italy), Piotr Pruszczyk (Poland), Marc Righini (Switzerland), Adam Torbicki (Poland), Eric Van Belle (France), José Luis Zamorano (Spain) Document Reviewers: Nazzareno Galié (CPG Review Coordinator) (Italy), J. Simon R. Gibbs (CPG Review Coordinator) (United Kingdom), Victor Aboyans (France), Walter Ageno (Italy), Stefan Agewall (Norway), Ana G. Almeida (Portugal), Felicita Andreotti (Italy), Emanuele Barbato (Italy), Johann Bauersachs (Germany), Andreas Baumbach (United Kingdom), Farzin Beygui (France), Jørn Carlsen (Denmark), Marco De Carlo (Italy), Marion Delcroix1 (Belgium), Victoria Delgado (Netherlands), Pilar Escribano Subias (Spain), Donna Fitzsimons (United Kingdom), -

Hospital Acquired Conditions Diagnostic Codes and Descriptions
HOSPITAL ACQUIRED CONDITIONS DIAGNOSTIC CODES AND DESCRIPTIONS DX DX LONG 630 Hydatidiform mole 631.0 Inappropriate change in quantitative human chorionic gonadotropin (hCG) in early pregnancy 631.8 Other abnormal products of conception 632 Missed abortion 633.00 Abdominal pregnancy without intrauterine pregnancy 633.01 Abdominal pregnancy with intrauterine pregnancy 633.10 Tubal pregnancy without intrauterine pregnancy 633.11 Tubal pregnancy with intrauterine pregnancy 633.20 Ovarian pregnancy without intrauterine pregnancy 633.21 Ovarian pregnancy with intrauterine pregnancy 633.80 Other ectopic pregnancy without intrauterine pregnancy 633.81 Other ectopic pregnancy with intrauterine pregnancy 633.90 Unspecified ectopic pregnancy without intrauterine pregnancy 633.91 Unspecified ectopic pregnancy with intrauterine pregnancy 634.00 Spontaneous abortion, complicated by genital tract and pelvic infection, unspecified 634.01 Spontaneous abortion, complicated by genital tract and pelvic infection, incomplete 634.02 Spontaneous abortion, complicated by genital tract and pelvic infection, complete 634.10 Spontaneous abortion, complicated by delayed or excessive hemorrhage, unspecified 634.11 Spontaneous abortion, complicated by delayed or excessive hemorrhage, incomplete 634.12 Spontaneous abortion, complicated by delayed or excessive hemorrhage, complete 634.20 Spontaneous abortion, complicated by damage to pelvic organs or tissues, unspecified 634.21 Spontaneous abortion, complicated by damage to pelvic organs or tissues, incomplete 634.22 -

ICD-10 to Deaths During Pregnancy, Childbirth and the Puerperium: ICD-MM
The WHO Application of ICD-10 to deaths during pregnancy, childbirth and the puerperium: ICD-MM The WHO Application of ICD-10 to deaths during pregnancy, childbirth and the puerperium: ICD-MM WHO Library Cataloguing-in-Publication Data The WHO application of ICD-10 to deaths during pregnancy, childbirth and puerperium: ICD MM. 1.Maternal mortality – classification. 2.Cause of death – classification. 3.Postpartum period. 4.Parturition. 5.Pregnancy complications – classification. 6.Pregnancy outcome. 7.Classification. 8.Manuals. I.World Health Organization. ISBN 978 92 4 154845 8 (NLM classification: WQ 270) © World Health Organization 2012 All rights reserved. Publications of the World Health Organization are available on the WHO web site (www.who.int) or can be purchased from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press through the WHO web site (http://www.who.int/about/licensing/ copyright_form/en/index.html). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. -

Complications of Pregnancy, Childbirth and the Puerperium Diagnosis Codes
Complications of Pregnancy, Childbirth and the Puerperium Diagnosis Codes 10058006 Miscarriage with amniotic fluid embolism (disorder) SNOMEDCT 10217006 Third degree perineal laceration (disorder) SNOMEDCT 102872000 Pregnancy on oral contraceptive (finding) SNOMEDCT 102873005 Pregnancy on intrauterine device (finding) SNOMEDCT 102875003 Surrogate pregnancy (finding) SNOMEDCT 102876002 Multigravida (finding) SNOMEDCT 106004004 Hemorrhagic complication of pregnancy (disorder) SNOMEDCT 106007006 Maternal AND/OR fetal condition affecting labor AND/OR delivery SNOMEDCT (disorder) 106008001 Delivery AND/OR maternal condition affecting management (disorder) SNOMEDCT 106009009 Fetal condition affecting obstetrical care of mother (disorder) SNOMEDCT 106010004 Pelvic dystocia AND/OR uterine disorder (disorder) SNOMEDCT 10853001 Obstetrical complication of general anesthesia (disorder) SNOMEDCT 111451002 Obstetrical injury to pelvic organ (disorder) SNOMEDCT 111452009 Postpartum afibrinogenemia with hemorrhage (disorder) SNOMEDCT 111453004 Retained placenta, without hemorrhage (disorder) SNOMEDCT 111454005 Retained portions of placenta AND/OR membranes without SNOMEDCT hemorrhage (disorder) 111458008 Postpartum venous thrombosis (disorder) SNOMEDCT 11209007 Cord entanglement without compression (disorder) SNOMEDCT 1125006 Sepsis during labor (disorder) SNOMEDCT 11454006 Failed attempted abortion with amniotic fluid embolism (disorder) SNOMEDCT 11687002 Gestational diabetes mellitus (disorder) SNOMEDCT 11942004 Perineal laceration involving pelvic -

Nonthrombotic Pulmonary Embolism
Eur Respir J 2009; 34: 452–474 DOI: 10.1183/09031936.00141708 CopyrightßERS Journals Ltd 2009 REVIEW Nonthrombotic pulmonary embolism P.G. Jorens*, E. Van Marck#, A. Snoeckx" and P.M. Parizel" ABSTRACT: Nonthrombotic pulmonary embolism (NTPE) is defined as embolisation to the AFFILIATIONS pulmonary circulation of different cell types (adipocytes, haematopoietic, amniotic, trophoblastic Depts of *Critical Care Medicine, #Pathology and or tumour), bacteria, fungi, foreign material or gas. The purpose of this article is to describe the "Radiology, UZA, Antwerp University clinical signs, pathogenesis, diagnosis and treatment of the different NTPE subtypes. Hospital, University of Antwerp, The complex and diverse pathogenesis of different subtypes of emboli is subject to continuing Belgium. speculation and is certainly far more complex than ‘‘simple’’ mechanical obstruction after CORRESPONDENCE embolisation of vascular thrombi. Nonthrombotic emboli may also lead to a severe inflammatory P.G. Jorens reaction both in the systemic and pulmonary circulation, as well as in the lung. UZA NTPE presents a formidable diagnostic challenge, as the condition often presents with very Antwerp University Hospital unusual and peculiar clinical signs that are frequently overlooked. They range from very dramatic University of Antwerp Wilrijkstraat 10 acute presentations such as acute respiratory distress syndrome to signs observed late in the B-2650 Edegem disease course. Pathological observations play a key role in the exact diagnosis, and sometimes Belgium carefully aspirated blood from the pulmonary artery or specific staining of cells recovered from E-mail: [email protected] bronchoalveolar lavage fluid may be helpful. Frequently, lung biopsies revealing severe Received: granulomatous reaction or unfortunate post-mortem pathological investigations of pulmonary Sept 15 2008 tissue are necessary to confirm the diagnosis. -
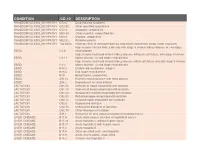
Condition Icd-10 Description
CONDITION ICD-10 DESCRIPTION RHABDOMYOLYSIS_MYOPATHY G72.0 Drug-induced myopathy RHABDOMYOLYSIS_MYOPATHY G72.89 Other specified myopathies RHABDOMYOLYSIS_MYOPATHY G72.9 Myopathy, unspecified RHABDOMYOLYSIS_MYOPATHY M60.80 Other myositis, unspecified site RHABDOMYOLYSIS_MYOPATHY M60.9 Myositis, unspecified RHABDOMYOLYSIS_MYOPATHY M62.82 Rhabdomyolysis RHABDOMYOLYSIS_MYOPATHY T46.6X5A Adverse effect of antihyperlipidemic and antiarteriosclerotic drugs, initial encounter Hypertensive chronic kidney disease with stage 5 chronic kidney disease or end stage ESRD I12.0 renal disease Hypertensive heart and chronic kidney disease without heart failure, with stage 5 chronic ESRD I13.11 kidney disease, or end stage renal disease Hypertensive heart and chronic kidney disease with heart failure and with stage 5 chronic ESRD I13.2 kidney disease, or end stage renal disease ESRD N18.5 Chronic kidney disease, stage 5 ESRD N18.6 End stage renal disease ESRD N19 Renal failure, unspecified ESRD Z91.15 Patient's noncompliance with renal dialysis ESRD Z99.2 Dependence on renal dialysis LACTATION O91.03 Infection of nipple associated with lactation LACTATION O91.13 Abscess of breast associated with lactation LACTATION O91.23 Nonpurulent mastitis associated with lactation LACTATION O92.03 Retracted nipple associated with lactation LACTATION O92.13 Cracked nipple associated with lactation LACTATION O92.5 Suppressed lactation LACTATION O92.70 Unspecified disorders of lactation LACTATION O92.79 Other disorders of lactation LACTATION Z39.1 Encounter for care and -

B Disorders of Autonomic Nervous System
Application of the International Classification of Diseases to Neurology \ Second Edition World Health Organization Geneva 1997 First edition 1987 Second edition 1997 Application of the international classification of diseases to neurology: ICD-NA- 2nd ed. 1. Neurology - classification 2. Nervous system diseases - classification I. Title: ICD-NA ISBN 92 4 154502 X (NLM Classification: WL 15) The World Health Organization welcomes requests for permission to reproduce or translate its publications, in part or in full. Applications and enquiries should be addressed to the Office of Publications, World Health Organization, Geneva, Switzerland, which will be glad to provide the latest information on any changes made to the text, plans for new editions, and reprints and translations already available. ©World Health Organization 1997 Publications of the World Health Organization enjoy copyright protection in accordance with the provisions of Protocol 2 of the Universal Copyright Convention. All rights reserved. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or of certain manufacturers' products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of -

CDC Cause of Death Classification
Florida PAMR Cause of Death Classification Underlying Cause of Death Pregnancy-Related Mortality Revised September 12, 2011 Florida’s classification is based on CDC’s Classification modified July 29, 2004. Florida’s version generally attempts to clarify and define the CDC classification to assure consistency and does not mean to alter the numbering system or the intended categorization process. All additions are highlighted. 00s+ CDC cause of death-hemorrhage and coagulopathies (COD1) Choose one: 01 Uterine laceration/rupture spont, forceps or TAB 02 Abruptio placentae 03 Placenta previa 10 Placenta acreta, percreta, or increta 04 Ruptured ectopic pregnancy 05 Uterine atony/postpartum bleeding, NOS 06 Uterine bleeding, NOS 07 Retained placenta/POC 08 Coagulopathies (including DIC) 18 Other (uterine artery lac, intra abdominal & other sites of hemorrhage) 19 Unknown 20s CDC cause of death-infection (COD2) Choose one: 20 Chorioamnionitis/antepartal infection 21 Postpartum pelvic infection 22 Generalized septicemia/septic shock, septic Ab 23 Peritonitis 24 Other pelvic tract infection (old version read "genital") 25 Nonpelvic infection (ex: pneumonia) 26 Urinary tract infections (ex. Pyelonephritis, cystitis, UTI) 28 Other 29 Unknown/NOS 30s CDC cause of death-Embolism (not cerebral) (COD3) Choose one: 30 Thrombotic (includes pulmonary embolism, NOS) 31 Amniotic fluid or Amniotic Anaphylactoid Syndrome, documented by autopsy/Swan Ganz 32 Amniotic fluid or Amniotic Anaphylactoid Syndrome, not documented by autopsy 33 Amniotic fluid or Amniotic -
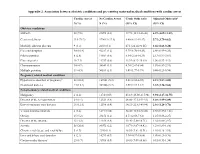
Appendix 2. Association Between Obstetric Conditions and Pre-Existing Maternal Medical Conditions with Cardiac Arrest
Appendix 2. Association between obstetric conditions and pre-existing maternal medical conditions with cardiac arrest Cardiac Arrest No Cardiac Arrest Crude Odds ratio Adjusted Odds ratioa N (%) N (%) (95% CI) (95% CI) Obstetric conditions Stillbirth 28 (9.8) 21492 (0.6) 17.91 (12.12-26.46) 6.87 (4.09-11.52) Cesarean delivery 215 (75.2) 979613 (27.5) 8.00 (6.12-10.47) 5.72 (3.79-8.62) Morbidly adherent placenta 9 (3.1) 2458 (0.1) 47.1 (24.24-91.66) 3.66 (1.63-8.20) Placental abruption 30 (10.4) 42217 (1.2) 9.79 (6.70-14.29) 2.48 (0.99-6.25) Polyhydramnios 8 (2.8) 19857 (0.6) 5.14 (2.55-10.39) 2.13 (0.97-3.67) Placenta previa 21(7.3) 21757 (0.6) 12.92 (8.29-20.15) 1.96 (0.93-4.13) Chorioamnionitis 14 (4.9) 38641 (1.1) 4.70 (2.47-8.04) 1.59 (0.87-2.91) Multiple gestation 13 (4.5) 54183 (1.5) 3.09 (1.77-5.39) 0.46 (0.23-0.94) Pregnancy related medical conditions Hypertensive disorders of pregnancyb 63 (22.0) 189861 (5.3) 5.03 (3.80-6.65) 2.18 (1.57-3.05) Gestational diabetes 44 (15.4) 204506 (5.7) 2.99 (2.17-4.12) 1.82 (1.26-2.63) Non-pregnancy related medical conditions Malignancy 4 (1.4) 1174 (0.03) 43.18 (16.08-115.94) 8.94 (2.17-36.75) Diseases of the nervous system 24 (8.4) 12521 (0.4) 26.02 (17.12-39.53) 3.48 (1.99-6.09) Lower respiratory tract diseases 36 (12.6) 12596 (0.4) 34.32 (23.63-49.84) 2.16 (1.24-3.76) Venous thromboembolism 4 (1.4) 1471 (0.04) 34.41 (12.81-92.42) 2.58 (0.59-11.22) Obesity 15 (5.2) 43151 (1.2) 4.53 (2.69-7.61) 1.25 (0.68-2.27) Diseases of the intestine 12 (4.2) 14834 (0.4) 10.49 (5.88-18.71) 1.42 (0.68-2.97)