Medicines in Pregnancy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guidelines for the Forensic Analysis of Drugs Facilitating Sexual Assault and Other Criminal Acts
Vienna International Centre, PO Box 500, 1400 Vienna, Austria Tel.: (+43-1) 26060-0, Fax: (+43-1) 26060-5866, www.unodc.org Guidelines for the Forensic analysis of drugs facilitating sexual assault and other criminal acts United Nations publication Printed in Austria ST/NAR/45 *1186331*V.11-86331—December 2011 —300 Photo credits: UNODC Photo Library, iStock.com/Abel Mitja Varela Laboratory and Scientific Section UNITED NATIONS OFFICE ON DRUGS AND CRIME Vienna Guidelines for the forensic analysis of drugs facilitating sexual assault and other criminal acts UNITED NATIONS New York, 2011 ST/NAR/45 © United Nations, December 2011. All rights reserved. The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. This publication has not been formally edited. Publishing production: English, Publishing and Library Section, United Nations Office at Vienna. List of abbreviations . v Acknowledgements .......................................... vii 1. Introduction............................................. 1 1.1. Background ........................................ 1 1.2. Purpose and scope of the manual ...................... 2 2. Investigative and analytical challenges ....................... 5 3 Evidence collection ...................................... 9 3.1. Evidence collection kits .............................. 9 3.2. Sample transfer and storage........................... 10 3.3. Biological samples and sampling ...................... 11 3.4. Other samples ...................................... 12 4. Analytical considerations .................................. 13 4.1. Substances encountered in DFSA and other DFC cases .... 13 4.2. Procedures and analytical strategy...................... 14 4.3. Analytical methodology .............................. 15 4.4. -

The In¯Uence of Medication on Erectile Function
International Journal of Impotence Research (1997) 9, 17±26 ß 1997 Stockton Press All rights reserved 0955-9930/97 $12.00 The in¯uence of medication on erectile function W Meinhardt1, RF Kropman2, P Vermeij3, AAB Lycklama aÁ Nijeholt4 and J Zwartendijk4 1Department of Urology, Netherlands Cancer Institute/Antoni van Leeuwenhoek Hospital, Plesmanlaan 121, 1066 CX Amsterdam, The Netherlands; 2Department of Urology, Leyenburg Hospital, Leyweg 275, 2545 CH The Hague, The Netherlands; 3Pharmacy; and 4Department of Urology, Leiden University Hospital, P.O. Box 9600, 2300 RC Leiden, The Netherlands Keywords: impotence; side-effect; antipsychotic; antihypertensive; physiology; erectile function Introduction stopped their antihypertensive treatment over a ®ve year period, because of side-effects on sexual function.5 In the drug registration procedures sexual Several physiological mechanisms are involved in function is not a major issue. This means that erectile function. A negative in¯uence of prescrip- knowledge of the problem is mainly dependent on tion-drugs on these mechanisms will not always case reports and the lists from side effect registries.6±8 come to the attention of the clinician, whereas a Another way of looking at the problem is drug causing priapism will rarely escape the atten- combining available data on mechanisms of action tion. of drugs with the knowledge of the physiological When erectile function is in¯uenced in a negative mechanisms involved in erectile function. The way compensation may occur. For example, age- advantage of this approach is that remedies may related penile sensory disorders may be compen- evolve from it. sated for by extra stimulation.1 Diminished in¯ux of In this paper we will discuss the subject in the blood will lead to a slower onset of the erection, but following order: may be accepted. -

Product Information
PRODUCT INFORMATION NAME OF THE MEDICINE POLARAMINE® (dexchlorpheniramine maleate) DESCRIPTION Polaramine (dexchlorpheniramine maleate) is the dextro-isomer of chlorpheniramine maleate. It is an antihistamine with anticholinergic properties Dexchlorpheniramine maleate (CAS no. 2438-32-6) is described chemically as (+)-2-[p- chloro-α-[2-(dimethylamino)ethyl]benzyl]pyridine maleate (1:1). It has the empirical formula of C16H19ClN2.C4H4O4 and the following structural formula: Dexchlorpheniramine maleate is a white, odourless, crystalline powder which in aqueous solution has a pH of between 4 and 5. It is freely soluble in water, soluble in alcohol and in chloroform, but only slightly soluble in benzene or ether. PHARMACOLOGY Pharmacodynamics Mechanism of Action: Dexchlorpheniramine, the d-isomer of the racemic compound chlorpheniramine, is two times more active than chlorpheniramine. Dexchlorpheniramine does not prevent the release of histamine, but rather, competes with free histamine for binding at the H1-receptor sites, and competitively antagonizes the effects of histamine on H1-receptors in the GI tract, uterus, large blood vessels, and bronchial muscle. Blockade of H1-receptors also suppresses the formation of oedema, flare, and pruritus that result from histaminic activity. Since dexchlorpheniramine binds to central and peripheral H1-receptors, sedative effects are likely to occur. H1-antagonists are structurally similar to anticholinergic agents and therefore possess the potential to exhibit anticholinergic properties of varying -

Report Update 1
Drug Class Review on Newer Antihistamines Final Report Update 1 April 2006 Original Report Date: November 2004 A literature scan of this topic is done periodically The purpose of this report is to make available information regarding the comparative effectiveness and safety profiles of different drugs within pharmaceutical classes. Reports are not usage guidelines, nor should they be read as an endorsement of, or recommendation for, any particular drug, use or approach. Oregon Health & Science University does not recommend or endorse any guideline or recommendation developed by users of these reports. Oregon Evidence-based Practice Center Oregon Health & Science University Mark Helfand, MD, MPH, Director Copyright © 2006 by Oregon Health & Science University Portland, Oregon 97201. All rights reserved. Note: A scan of the medical literature relating to the topic is done periodically (see http://www.ohsu.edu/ohsuedu/research/policycenter/DERP/about/methods.cfm for scanning process description). Upon review of the last scan, the Drug Effectiveness Review Project governance group elected not to proceed with another full update of this report. Some portions of the report may not be up to date. Prior version of this report can be accessed at the DERP website. Final Report Update #1 Drug Effectiveness Review Project TABLE OF CONTENTS INTRODUCTION.............................................................................................................................................................4 SCOPE AND KEY QUESTIONS.............................................................................................................................6 -

Programme & Abstracts
The 57th Annual Meeting of the International Association of Forensic Toxicologists. 2nd - 6th September 2019 BIRMINGHAM, UK The ICC Birmingham Broad Street, Birmingham B1 2EA Programme & Abstracts 1 Thank You to our Sponsors PlatinUm Gold Silver Bronze 2 3 Contents Welcome message 5 Committees 6 General information 7 iCC maps 8 exhibitors list 10 Exhibition Hall 11 Social Programme 14 opening Ceremony 15 Schedule 16 Oral Programme MONDAY 2 September 19 TUESDAY 3 September 21 THURSDAY 5 September 28 FRIDAY 6 September 35 vendor Seminars 42 Posters 46 oral abstracts 82 Poster abstracts 178 4 Welcome Message It is our great pleasure to welcome you to TIAFT Gala Dinner at the ICC on Friday evening. On the accompanying pages you will see a strong the UK for the 57th Annual Meeting of scientific agenda relevant to modern toxicology and we The International Association of Forensic thank all those who submitted an abstract and the Toxicologists Scientific Committees for making the scientific programme (TIAFT) between 2nd and 6th a success. Starting with a large Young Scientists September 2019. Symposium and Dr Yoo Memorial plenary lecture by Prof Tony Moffat on Monday, there are oral session topics in It has been decades since the Annual Meeting has taken Clinical & Post-Mortem Toxicology on Tuesday, place in the country where TIAFT was founded over 50 years Human Behaviour Toxicology & Drug-Facilitated Crime on ago. The meeting is supported by LTG (London Toxicology Thursday and Toxicology in Sport, New Innovations and Group) and the UKIAFT (UK & Ireland Association of Novel Research & Employment/Occupational Toxicology Forensic Toxicologists) and we thank all our exhibitors and on Friday. -

Pregnancy-Safe-Medications
PEACHTREE WOMEN’S SPECIALISTS, P.C. Obstetrics and Gynecology Melissa Counihan, M.D Bonita Dozier, M.D. James P. Ingvoldstad, MD James Knoer, M.D. Helen McSwain, M.D. Archie Roberts, M.D. Lillian Schapiro, MD PREGNANCY SAFE MEDICATIONS Antihistamines o Actifed o Benadryl o Claritin o Claritin-D o Chlor-Trimeton-D o Chlor-Trimeton-DM o Sudafed* o Zyrtec and Zyrtec-D * Georgia law now requires that Sudafed be requested from the pharmacist, even though it is not a prescription medication. Cough o Robitussin o Robitussin DM o Robitussin PE* o You may take any cough drop *DO NOT use if taking Sudafed or Brethine/Terbutaline. Calcium Supplement o Tums EX- two tablets twice daily o Viactiv Constipation o Colace o Fibercon o Konsyl o Metamucil o Milk of Magnesia o Perdiem Increase dietary roughage, bran, dark green leafy vegetables and fruits. Drink eight to ten glasses of water daily. Decongestants* o Sudafed o Sudafed Sinus o Sudafed Non-drying o Actifed o Tylenol Sinus o Benadryl o Doxylamine Succinate *After the first trimester PREGNANCY SAFE MEDICATIONS (Continued) Dry Skin o Cocoa Butter o Eucerin Lotion o Vitamin E Lotion Fever o Tylenol* o Tylenol Extra Strength* *DO NOT take more than 12 tablets of Regular Strength Tylenol in 24 hours and no more than 6 tablets of Extra Strength Tylenol in 24 hours. Gas o Mylicon o Mylanta GAS o Phazyme o Mylanta Antacid/Anti-gas Hemorrhoids o Preparation H o Colace o Annusol Suppository/Ointment (With or without Cortisone) Increase dietary roughage, bran, dark green leafy vegetables and fruits. -
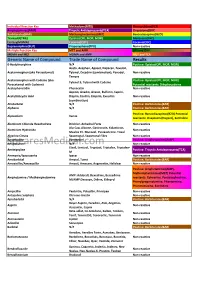
Cross Reaction Guide
Individual Reaction Key Methadone(MTD) Phencyclidine(PCP) Amphetamines(AMP) Tricyclic Antidepressants(TCA) Oxycodone(OXY) Barbiturates(BAR) Methamphetamines(MET) Benzodiazepines(BZO) Tramadol(TML) Opiates(OPI, MOP, MOR) Marijuana(THC) Ecstasy(MDMA) Cotinine(COT) Cocaine(COC) Buprenorphine(BUP) Propoxyphene(PPX) Non-reactive Multiple Reaction Key MET and AMP OPI and OXY MDMA and MET MDMA and AMP MET and TCA Generic Name of Compound Trade Name of Compound Results 6-Acetylmorphine N/A Positive: Opiates(OPI, MOP, MOR) Aceta, Acephen, Apacet, Dapacen, Feverall, Acetaminophen (aka Paracetamol) Tylenol, Excedrin (combination), Panadol, Non-reactive Tempra Acetaminophen with Codeine (aka Positive: Opiates(OPI, MOP, MOR) Tylenol 3, Tylenol with Codeine Paracetamol with Codeine) Potential reactants: Dihydrocodeine Acetophenetidin Phenacetin Non-reactive Aspirin, Anadin, Anasin, Bufferin, Caprin, Acetylsalicyclic Acid Disprin, Ecotrin, Empirin, Excedrin Non-reactive (combination) Allobarbital N/A Positive: Barbiturates(BAR) Alphenol N/A Positive: Barbiturates(BAR) Positive: Benzodiazepines(BZO) Potential Alprazolam Xanax reactants: Oxaprozin (Daypro), Sertraline Aluminum Chloride Hexahydrate Drichlor, Anhydrol Forte Non-reactive Alu-Cap, Alisone, Gastrocote, Kolanticon, Aluminum Hydroxide Non-reactive Maalox TC, Mucogel, Pyrogastrone, Topal Alverine Citrate Spasmonal, Spasmonal Fibre Non-reactive Amantadine Symmetrel Positive: Amphetamines(AMP) SpearesMedical.comAminopyrine N/A Non-reactive Elavil, Lentizol, Tryptizol, Triptafen, Triptafen- Amitriptyline -

References to Argentina
References to Argentina Part 1 RECENT STATISTICS AND TREND ANALYSIS OF ILLICIT DRUG MARKETS A. EXTENT OF ILLICIT DRUG USE AND HEALTH CONSEQUENCES The Americas (pages 12 to 14 WDR 13) In the Americas, a high prevalence of most illicit drugs, essentially driven by estimates in North America, was observed, with the prevalence of cannabis (7.9 per cent) and cocaine (1.3 per cent) being particularly high in the region. South America, Central America and the Caribbean The annual prevalence of cocaine use in South America (1.3 per cent of the adult population) is comparable to levels in North America, while it remains much higher than the global average in Central America (0.6 per cent) and the Caribbean (0.7 per cent). Cocaine use has increased significantly in Brazil, Costa Rica and, to lesser extent, Peru while no change in its use was reported in Argentina. The use of cannabis in South America is higher (5.7 per cent) than the global average, but lower in Central America and Caribbean (2.6 and 2.8 per cent respectively). In South America and Central America the use of opioids (0.3 and 0.2 per cent, respectively) and Ecstasy (0.1 per cent each) also remain well below the global average. While opiates use remains low, countries such as Colombia report that heroin use is becoming increasingly common among certain age groups and socio-economic classes.30 Part 2 NEW PSYCHOACTIVE SUBSTANCES C. THE RECENT EMERGENCE AND SPREAD OF NEW PSYCHOACTIVE SUBSTANCES (page 67 WDR 13) Spread at the global level Number of countries reporting the emergence of new psychoactive substances Pursuant to Commission on Narcotic Drugs resolution 55/1, entitled “Promoting international cooperation in responding to the challenges posed by new psychoactive substances”, in 2012 UNODC sent a questionnaire on NPS to all Member States, to which 80 countries and territories replied. -

(12) United States Patent (10) Patent No.: US 7,863,296 B2 Weiner Et Al
USOO7863296B2 (12) United States Patent (10) Patent No.: US 7,863,296 B2 Weiner et al. (45) Date of Patent: *Jan. 4, 2011 (54) SELECTIVE SEROTONIN RECEPTOR 5,795,894. A 8, 1998 Shue et al. NVERSEAGONSTS AS THERAPEUTICS 5,869,488 A 2f1999 Shue et al. FOR DISEASE 5,874.445 A * 2/1999 Carr et al. ................... 514,317 5,877,173 A 3/1999 Olney et al. 5,912,132 A 6/1999 Brann (75) Inventors: David M. Weiner, San Diego, CA (US); 5,952.324 A 9/1999 Barbachyn et al. Robert E. Davis, San Diego, CA (US); 5,955,281 A 9, 1999 Brann Mark R. Brann, Del Mar, CA (US); 6,107,324 A 8, 2000 Behan et al. Anna-Maria Frost-Jensen, legal 6,140,509 A 10/2000 Behan et al. representative, Rye, NH (US); Norman 6,150,393 A 11/2000 Behan et al. Nash, Carlsbad, CA (US) 6,358,698 B1 3/2002 Weiner et al. 6.479,480 B1 1 1/2002 Moyes et al. (73) Assignee: ACADIA Pharmaceuticals, Inc., San 6,486,153 B1 1 1/2002 Castro Pineiro et al. Diego, CA (US) 6,617.339 B1 9, 2003 Gravestock 6,756,393 B2 6/2004 Andersson et al. (*) Notice: Subject to any disclaimer, the term of this 6,815,458 B2 11/2004 Andersson et al. 6,911,452 B2 6/2005 Schlienger patent is extended or adjusted under 35 7,022,698 B2 4/2006 Hamied et al. U.S.C. 154(b) by 1393 days. -

Doxylamine Succinate and Pyridoxine Hydrochloride (Diclegis®, Bonjesta®) Duration Limit
Cigna National Formulary Coverage Policy Drug Quantity Management – Per Days Doxylamine succinate and pyridoxine hydrochloride (Diclegis®, Bonjesta®) Duration Limit Table of Contents Product Identifer(s) National Formulary Medical Necessity ................ 1 60889 Conditions Not Covered....................................... 2 Background .......................................................... 2 References .......................................................... 2 Revision History ................................................... 2 INSTRUCTIONS FOR USE The following Coverage Policy applies to health benefit plans administered by Cigna Companies. Certain Cigna Companies and/or lines of business only provide utilization review services to clients and do not make coverage determinations. References to standard benefit plan language and coverage determinations do not apply to those clients. Coverage Policies are intended to provide guidance in interpreting certain standard benefit plans administered by Cigna Companies. Please note, the terms of a customer’s particular benefit plan document [Group Service Agreement, Evidence of Coverage, Certificate of Coverage, Summary Plan Description (SPD) or similar plan document] may differ significantly from the standard benefit plans upon which these Coverage Policies are based. For example, a customer’s benefit plan document may contain a specific exclusion related to a topic addressed in a Coverage Policy. In the event of a conflict, a customer’s benefit plan document always supersedes the information in the Coverage Policies. In the absence of a controlling federal or state coverage mandate, benefits are ultimately determined by the terms of the applicable benefit plan document. Coverage determinations in each specific instance require consideration of 1) the terms of the applicable benefit plan document in effect on the date of service; 2) any applicable laws/regulations; 3) any relevant collateral source materials including Coverage Policies and; 4) the specific facts of the particular situation. -

Doxylamine Succinate-Pyridoxine Hydrochloride
Doxylamine succinate-pyridoxine hydrochloride This sheet is about exposure to doxylamine succinate-pyridoxine hydrochloride in pregnancy and while breastfeeding. This information should not take the place of medical care and advice from your healthcare providers. What is doxylamine succinate-pyridoxine hydrochloride? The combination of 10mg of doxylamine succinate and 10mg of pyridoxine hydrochloride is a medication used to treat nausea and vomiting of pregnancy (NVP), also called “morning sickness.” For more information on NVP, please see the MotherToBaby fact sheet Nausea and Vomiting of Pregnancy (https://mothertobaby.org/fact-sheets/nausea-vomiting-pregnancy-nvp/pdf/). Doxylamine succinate is an antihistamine. Antihistamines lessen the symptoms of allergic reactions and colds and help to treat insomnia (hard time sleeping). Pyridoxine hydrochloride is a form of vitamin B6. In the United States, the combination of doxylamine and pyridoxine has been sold under the name Diclegis® since 2013. In Canada, it has been sold under the brand name Diclectin®. Diclegis® and Diclectin® are delayed-release tablets available by prescription. Delayed-release means that the tablet coating prevents the ingredients from being absorbed too quickly by the body. Doxylamine succinate and/or pyridoxine hydrochloride may also be available as over-the- counter medicines. I take doxylamine succinate-pyridoxine hydrochloride. Can it make it harder for me to get pregnant? Based on the data available, it is not known if doxylamine succinate-pyridoxine hydrochloride can make it harder to become pregnant. I just found out I am pregnant. Should I stop taking doxylamine succinate-pyridoxine hydrochloride? Talk with your healthcare providers before making any changes to how you take your medication(s). -

HANDBOOK of Medicinal Herbs SECOND EDITION
HANDBOOK OF Medicinal Herbs SECOND EDITION 1284_frame_FM Page 2 Thursday, May 23, 2002 10:53 AM HANDBOOK OF Medicinal Herbs SECOND EDITION James A. Duke with Mary Jo Bogenschutz-Godwin Judi duCellier Peggy-Ann K. Duke CRC PRESS Boca Raton London New York Washington, D.C. Peggy-Ann K. Duke has the copyright to all black and white line and color illustrations. The author would like to express thanks to Nature’s Herbs for the color slides presented in the book. Library of Congress Cataloging-in-Publication Data Duke, James A., 1929- Handbook of medicinal herbs / James A. Duke, with Mary Jo Bogenschutz-Godwin, Judi duCellier, Peggy-Ann K. Duke.-- 2nd ed. p. cm. Previously published: CRC handbook of medicinal herbs. Includes bibliographical references and index. ISBN 0-8493-1284-1 (alk. paper) 1. Medicinal plants. 2. Herbs. 3. Herbals. 4. Traditional medicine. 5. Material medica, Vegetable. I. Duke, James A., 1929- CRC handbook of medicinal herbs. II. Title. [DNLM: 1. Medicine, Herbal. 2. Plants, Medicinal.] QK99.A1 D83 2002 615′.321--dc21 2002017548 This book contains information obtained from authentic and highly regarded sources. Reprinted material is quoted with permission, and sources are indicated. A wide variety of references are listed. Reasonable efforts have been made to publish reliable data and information, but the author and the publisher cannot assume responsibility for the validity of all materials or for the consequences of their use. Neither this book nor any part may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, microfilming, and recording, or by any information storage or retrieval system, without prior permission in writing from the publisher.