Deleterious Effects of IL-9–Activated Mast Cells and Neuroprotection by Antihistamine Drugs in the Developing Mouse Brain
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Product Information
PRODUCT INFORMATION NAME OF THE MEDICINE POLARAMINE® (dexchlorpheniramine maleate) DESCRIPTION Polaramine (dexchlorpheniramine maleate) is the dextro-isomer of chlorpheniramine maleate. It is an antihistamine with anticholinergic properties Dexchlorpheniramine maleate (CAS no. 2438-32-6) is described chemically as (+)-2-[p- chloro-α-[2-(dimethylamino)ethyl]benzyl]pyridine maleate (1:1). It has the empirical formula of C16H19ClN2.C4H4O4 and the following structural formula: Dexchlorpheniramine maleate is a white, odourless, crystalline powder which in aqueous solution has a pH of between 4 and 5. It is freely soluble in water, soluble in alcohol and in chloroform, but only slightly soluble in benzene or ether. PHARMACOLOGY Pharmacodynamics Mechanism of Action: Dexchlorpheniramine, the d-isomer of the racemic compound chlorpheniramine, is two times more active than chlorpheniramine. Dexchlorpheniramine does not prevent the release of histamine, but rather, competes with free histamine for binding at the H1-receptor sites, and competitively antagonizes the effects of histamine on H1-receptors in the GI tract, uterus, large blood vessels, and bronchial muscle. Blockade of H1-receptors also suppresses the formation of oedema, flare, and pruritus that result from histaminic activity. Since dexchlorpheniramine binds to central and peripheral H1-receptors, sedative effects are likely to occur. H1-antagonists are structurally similar to anticholinergic agents and therefore possess the potential to exhibit anticholinergic properties of varying -

Report Update 1
Drug Class Review on Newer Antihistamines Final Report Update 1 April 2006 Original Report Date: November 2004 A literature scan of this topic is done periodically The purpose of this report is to make available information regarding the comparative effectiveness and safety profiles of different drugs within pharmaceutical classes. Reports are not usage guidelines, nor should they be read as an endorsement of, or recommendation for, any particular drug, use or approach. Oregon Health & Science University does not recommend or endorse any guideline or recommendation developed by users of these reports. Oregon Evidence-based Practice Center Oregon Health & Science University Mark Helfand, MD, MPH, Director Copyright © 2006 by Oregon Health & Science University Portland, Oregon 97201. All rights reserved. Note: A scan of the medical literature relating to the topic is done periodically (see http://www.ohsu.edu/ohsuedu/research/policycenter/DERP/about/methods.cfm for scanning process description). Upon review of the last scan, the Drug Effectiveness Review Project governance group elected not to proceed with another full update of this report. Some portions of the report may not be up to date. Prior version of this report can be accessed at the DERP website. Final Report Update #1 Drug Effectiveness Review Project TABLE OF CONTENTS INTRODUCTION.............................................................................................................................................................4 SCOPE AND KEY QUESTIONS.............................................................................................................................6 -

Antihistamine Therapy in Allergic Rhinitis
CLINICAL REVIEW Antihistamine Therapy in Allergic Rhinitis Paul R. Tarnasky, MD, and Paul P. Van Arsdel, Jr, MD Seattle, Washington Allergic rhinitis is a common disorder that is associated with a high incidence of mor bidity and considerable costs. The symptoms of allergic rhinitis are primarily depen dent upon the tissue effects of histamine. Antihistamines are the mainstay of therapy for allergic rhinitis. Recently, a second generation of antihistamines has become available. These agents lack the adverse effect of sedation, which is commonly associated with older antihistamines. Current practice of antihistamine therapy in allergic rhinitis often involves random selection among the various agents. Based upon the available clinical trials, chlorpheniramine appears to be the most reasonable initial antihistaminic agent. A nonsedating antihis tamine should be used initially if a patient is involved in activities where drowsiness is dangerous. In this comprehensive review of allergic rhinitis and its treatment, the cur rent as well as future options in antihistamine pharmacotherapy are emphasized. J Fam Pract 1990; 30:71-80. llergic rhinitis is a common condition afflicting some defined by the period of exposure to those agents to which A where between 15 and 30 million people in the United a patient is sensitive. Allergens in seasonal allergic rhinitis States.1-3 The prevalence of disease among adolescents is consist of pollens from nonflowering plants such as trees, estimated to be 20% to 30%. Two thirds of the adult grasses, and weeds. These pollens generally create symp allergic rhinitis patients are under 30 years of age.4-6 Con toms in early spring, late spring through early summer, sequently, considerable costs are incurred in days lost and fall, respectively. -

Trends in Prescription Medication Use Among Children and Adolescents— United States, 1999-2014
Supplementary Online Content Hales CM, Kit BK, Gu Q, Ogden CL. Trends in prescription medication use among children and adolescents— United States, 1999-2014. JAMA. doi:10.1001/jama.2018.5690 eTable. Classification of Prescription Medications Reported by NHANES Participants Aged 0-19 Years From 1999- 2000 to 2013-2014 by Therapeutic Class This supplementary material was provided by the authors to give readers additional informtion about their work. © 2018 American Medical Association. All rights reserved. Downloaded From: https://jamanetwork.com/ on 09/27/2021 eTable. Classification of Prescription Medications Reported by NHANES Participants Aged 0-19 Years From 1999- 2000 to 2013-2014 by Therapeutic Class Therapeutic classes are based on the Lexicon Plus prescription medication database and only those classes reported in the manuscript are listed. ADHD Medications Antiadrenergic Agents, Centrally Acting Clonidine Guanfacine CNS Stimulants Amphetamines Amphetamine Amphetamine; Dextroamphetamine Dextroamphetamine Lisdexamfetamine Methylphenidate or Dexmethylphenidate Dexmethylphenidate Methylphenidate Other CNS Stimulant Pemoline Selective Norepinephrine Reuptake Inhibitor Atomoxetine Antibiotics Cephalosporins Cefadroxil Cephalexin Cefaclor Cefprozil Cefuroxime Loracarbef Cefdinir Cefditoren Cefixime Cefpodoxime Ceftibuten Ceftriaxone Glycopeptide Antibiotics Vancomycin H. Pylori Eradication Agents Amoxicillin; Clarithromycin; Lansoprazole Lincomycin Derivatives Clindamycin Macrolide Derivatives Telithromycin Azithromycin Clarithromycin -

POLARAMINE® Repetabs (Red and Colour Free), Tablets (Red and Colour Free) and Syrup Dexchlorpheniramine Maleate
POLARAMINE® Repetabs (Red and Colour Free), Tablets (Red and Colour Free) and Syrup Dexchlorpheniramine maleate Consumer Medicine Information What is in this leaflet? Polaramine can also be used to treat • You are also taking medicines drug reactions. used to treat depression such as monoamine oxidase inhibitor This leaflet answers some common Polaramine belongs to a class of (MAOI). questions about Polaramine. It does medicines known as antihistamines. not contain all of the available Taking Polaramine together with Antihistamines help reduce allergic information. It does not take the a MAOI may exaggerate the symptoms by preventing the effects place of talking to your doctor or effects of Polaramine and cause a of a substance called histamine. pharmacist. severe drop in your blood Histamine is produced by the body in pressure. All medicines have risks and response to foreign substances which benefits. Your doctor or pharmacist the body is allergic to. Do not give Polaramine Syrup to has weighed the risks of you taking children under 2 years of age. Polaramine against the benefits they Your doctor or pharmacist may have Do not give Polaramine Tablets expect it will have for you. prescribed Polaramine for another reason. and Repetabs to children less than If you have any concerns about 12 years. taking this medicine, ask your Ask your doctor or pharmacist if Do not take Polaramine after the doctor or pharmacist. you have any questions about why Polaramine has been prescribed expiry date (EXP) printed on the Keep this leaflet with the medicine. for you. pack. You may need to read it again. -
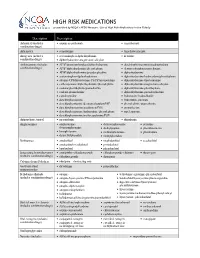
HIGH RISK MEDICATIONS As Specified by NCQA’S HEDIS Measure: Use of High Risk Medications in the Elderly
HIGH RISK MEDICATIONS as specified by NCQA’s HEDIS Measure: Use of High Risk Medications in the Elderly Description Prescription Antianxiety (includes • aspirin-meprobamate • meprobamate combination drugs) Antiemetics • scopolamine • trimethobenzamide Analgesics (includes • acetaminophen-diphenhydramine • ketorolac combination drugs) • diphenhydramine-magnesium salicylate Antihistamines (includes • APAP/dextromethorphan/diphenhydramine • dexchlorpheniramine-pseudoephedrine combination drugs) • APAP/diphenhydramine/phenylephrine • dextromethorphan-promethazine • APAP/diphenhydramine/pseudoephedrine • diphenhydramine • acetaminophen-diphenhydramine • diphenhydramine/hydrocodone/phenylephrine • atropine/CPM/hyoscyamine/PE/PPA/scopolamine • diphenhydramine-tripelennamine • carbetapentane/diphenhydramine/phenylephrine • diphenhydramine-magnesium salicylate • codeine/phenylephrine/promethazine • diphenhydramine-phenylephrine • codeine-promethazine • diphenhydramine-pseudoephedrine • cyproheptadine • hydroxyzine hydrochloride • dexchlorpheniramine • hydroxyzine pamoate • dexchlorpheniramine/dextromethorphan/PSE • phenylephrine-promethazine • dexchlorpheniramine/guaifenesin/PSE • promethazine • dexchlorpheniramine/hydrocodone/phenylephrine • tripelennamine • dexchlorpheniramine/methscopolamine/PSE Antipsychotic, typical • mesoridazine • thioridazine Amphetamines • amphetamine- • dextroamphetamine • pemoline dextroamphetamine • diethylpropion • phendimetrazine • benzphetamine • methamphetamine • phentermine • dexmethylphenidate • methylphenidate -

Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DIX to the HTSUS—Continued
20558 Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DEPARMENT OF THE TREASURY Services, U.S. Customs Service, 1301 TABLE 1.ÐPHARMACEUTICAL APPEN- Constitution Avenue NW, Washington, DIX TO THE HTSUSÐContinued Customs Service D.C. 20229 at (202) 927±1060. CAS No. Pharmaceutical [T.D. 95±33] Dated: April 14, 1995. 52±78±8 ..................... NORETHANDROLONE. A. W. Tennant, 52±86±8 ..................... HALOPERIDOL. Pharmaceutical Tables 1 and 3 of the Director, Office of Laboratories and Scientific 52±88±0 ..................... ATROPINE METHONITRATE. HTSUS 52±90±4 ..................... CYSTEINE. Services. 53±03±2 ..................... PREDNISONE. 53±06±5 ..................... CORTISONE. AGENCY: Customs Service, Department TABLE 1.ÐPHARMACEUTICAL 53±10±1 ..................... HYDROXYDIONE SODIUM SUCCI- of the Treasury. NATE. APPENDIX TO THE HTSUS 53±16±7 ..................... ESTRONE. ACTION: Listing of the products found in 53±18±9 ..................... BIETASERPINE. Table 1 and Table 3 of the CAS No. Pharmaceutical 53±19±0 ..................... MITOTANE. 53±31±6 ..................... MEDIBAZINE. Pharmaceutical Appendix to the N/A ............................. ACTAGARDIN. 53±33±8 ..................... PARAMETHASONE. Harmonized Tariff Schedule of the N/A ............................. ARDACIN. 53±34±9 ..................... FLUPREDNISOLONE. N/A ............................. BICIROMAB. 53±39±4 ..................... OXANDROLONE. United States of America in Chemical N/A ............................. CELUCLORAL. 53±43±0 -

Technical File Or Summary of the Characteristics of The
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Dexchlorpheniramine Maleate 5mg/ml solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of Dexchlorpheniramine Maleate contains 5 mg of Dexchlorpheniramine Maleate. For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Solution for injection Clear and particle free solution for injection. 4. CLINICAL PARTICULARS 4.1. Therapeutic indications Symptomatic treatment of acute urticaria when oral administration is not possible. Dexchlorpheniramine Maleate 5mg/ml solution for injection is indicated in adults and children older of 30 months. 4.2. Posology and method of administration Posology Adults: The dose should be personalised according to the requirements and response of the patient. Elderly patients: Dexchlorpheniramine Maleate 5mg/ml should be used with precaution in elderly patients (See section 4.4). Renal and hepatic impairment: Dexchlorpheniramine Maleate 5mg/ml should be used with precaution (See section 4.4). Paediatric population: The safety and efficacy of Dexchlorpheniramine Maleate 5mg/ml solution for injection in children aged less than 30 months has not yet been established. No data are available. Method of administration 1 The recommended dose is 5 mg (1 ampoule) administered intravenously or intramuscularly. The maximum daily dose is of 20 mg (4 ampoules). In the case of a reaction during a transfusion, do not administer Dexchlorpheniramine Maleate 5mg/ml solution for injection in the transfusion, but apart. 4.3. Contraindications -Hypersensitivity to the active substance, or any of the excipients listed in section 6.1, or to other antihistamines of similar chemical structure. -Children under 30 months of age (See section 4.4). -

Medicines in Pregnancy
MEDICINES IN PREGNANCY Most medicines used during pregnancy will cross the placenta and reach the baby. Some may be harmful to you or your baby so it is important to seek the advice of your doctor or healthcare provider before you start, change or stop taking medicines. This factsheet contains general advice only; it is not intended to replace the individual care and advice of your healthcare provider. It does not include information about all side effects and should be read together with the product information provided with medicines. What are medicines? What can I take for aches and pain? Medicines are preparations used for the treatment or You may use paracetamol during pregnancy at the prevention of disease. Medicines include: recommended dose to treat mild to moderate pain, • Prescription medicine - prescribed by doctors, such as headache, toothache, muscular pain or to dentists or other health professionals. reduce fever.1,2 • Over-the-counter (OTC) medicine - purchased over- Nonsteroidal anti-inflammatory drugs (NSAIDs) such the-counter at pharmacies, supermarkets or health as aspirin, ibuprofen and diclofenac should be avoided food stores. in pregnancy, especially after 30 weeks because they *For information on the use of herbal and traditional medicines can be harmful to your baby. Talk to your doctor or please refer to the Herbal Medicines in Pregnancy and Breastfeeding factsheet. pharmacist before taking any NSAIDs during pregnancy.3,4 Is it safe to use prescription medicine while I am pregnant? What can I take for allergies and hayfever? Some women have conditions which need ongoing Allergies and hayfever symptoms include a runny and treatment, while others may develop or experience new blocked nose, sneezing, itching of the nose, eyes, ears conditions, such as morning sickness or heartburn, or throat and watery, red irritated eyes. -

First-Generation Antihistamines (Medications Commonly Used To
Highrisk medication reference sheet The Pharmacy Quality Alliance has determined the following medications have the highest risk of side effects among those 65 years of age or older. “Highrisk” means a medicine can cause serious health problems or accidents. Highrisk medications can be: • A medicine that raises your risk of drowsiness, confusion, depression, organ damage, serious harm from a fall, or other dangerous side effects. • A medicine for one health problem that worsens another health problem. • Two or more medications that are dangerous when taken together. The more medicines you take, the greater the risk of negative interactions. Please review the list of highrisk medications below. If you are taking one or more of the medications listed, please speak with your doctor to determine if there are safer choices with fewer possible side effects. Firstgeneration antihistamines (medications commonly used to treat allergies) • Brompheniramine • Cyproheptadine • Hydroxyzine • Carbinoxamine • Dexchlorpheniramine • Promethazine • Chlorpheniramine • Diphenhydramine (oral) • Triprolidine • Clemastine • Doxylamine AntiParkinson agents (to treat Parkinson’s disease) • Benztropine (oral) • Trihexyphenidyl Antithrombotics (medications used to prevent blood from clotting inappropriately) • Ticlopidine • Dipyridamole Antiinfective (medication used to treat infections) • Nitrofurantoin (only when taken for 90 days or more) Alpha blockers (medications that help blood vessels remain open) • Guanfacine • Reserpine (only if you take more than -

The Relation Between Antihistamine Medication During Early Pregnancy & Birth Defects
The Egyptian Journal of Medical Human Genetics (2015) 16, 287–290 HOSTED BY Ain Shams University The Egyptian Journal of Medical Human Genetics www.ejmhg.eg.net www.sciencedirect.com REVIEW The relation between antihistamine medication during early pregnancy & birth defects Rabah M. Shawky a,*, Neveen S. Seifeldin b a Genetics Unit, Pediatric Department, Ain Shams University, Egypt b Dermatology, Venereology and Andrology Department, Ain Shams University, Egypt Received 6 April 2015; accepted 16 April 2015 Available online 11 May 2015 KEYWORDS Abstract Antihistamines are a group of medications which can inhibit various histaminic actions Antihistamine; at one of two histamine receptors (H1 or H2). H1 receptor antagonists are used for the relief of Birth defects; allergic dermatological and nondermatological conditions. We will review classes of antihistamines Congenital malformation; (H1 antagonists) and the relationship between specific antihistamines and specific birth defects. H1 antagonist; Although many findings provide reassurance about the relative safety of many antihistamine drugs Early pregnancy; and that any malformation reported is most probably caused by chance, studies are still required to Dermatological conditions assure fetal safety. As pruritus is sometimes troublesome for pregnant women topical medications like emollients should be tried first in the first trimester of pregnancy. Also pregnant women should be advised to consult their health care provider before taking any medication. Ó 2015 Production and hosting by Elsevier B.V. on behalf of Ain Shams University. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/). Contents 1. Introduction . 287 2. Classification of H1 antihistamines . -

High-Risk Medications in the Elderly
High-Risk Medications in the Elderly The Centers for Medicare & Medicaid Services (CMS) contracted with the National Committee for Quality Assurance (NCQA) to develop clinical strategies to monitor and evaluate the quality of care provided to Medicare beneficiaries. The NCQA’s Geriatric Measurement Advisory Panel identified several categories of medications that have an increased risk of adverse effects to elderly patients. The enclosed chart identifies several key medication categories that CMS and NCQA are monitoring. In an effort to ensure patients’ safety, many of our clients have established pre-authorization protocols for those prescriptions for high risk medications in patients older than 65 years of age. Since pharmacists have a very important role in patient care, we want you to be part of this safety initiative. We strongly encourage that you contact the prescriber when your elderly patient is requesting a new or refilled prescription of a high-risk medication listed on the below chart. Category High Risk Medications Alternatives Analgesics butalbital/APAP Mild Pain: butalbital/APAP/caffeine (ESGIC, FIORICET) acetaminophen, codeine, short-term NSAIDs butalbital /APAP/caffeine/codeine Moderate/Severe Pain: butalbital/ASA/caffeine (FIORINAL) tramadol (ULTRAM), tramadol/APAP* (ULTRACET), butalbital/ASA/caffeine/codeine morphine sulfate (MS CONTIN), ketorolac (TORADOL) hydrocodone/APAP (VICODIN, etc.), oxycodone indomethacin (INDOCIN) (OXYIR), oxycodone/APAP (PERCOCET), fentanyl meperidina (DEMEROL) patch (DURAGESIC), OXYCONTIN