Caverjectimpulseinj.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
What Is a Hydrocelectomy, Spermatocelectomy and Epididymal Cystectomy? a Hydrocele Is an Abnormal Fluid Collection Between the Outer Tissue Layers of the Testicle
Dr. Kevin G. Kwan, BSc (Hons), MD, FRCS(C) Minimally Invasive Surgery and General Urology Assistant Clinical Professor Division of Urology, Department of Surgery McMaster University Chief of Surgery, Milton District Hospital Georgetown Hospital • Milton District Hospital • Oakville Trafalgar Memorial Hospital Suite 205 - 311 Commercial Street • Milton • Ontario • L9T 3Z9 • Tel: (905) 875-3920 • Fax: (905) 875-4340 Email: [email protected] • Web: www.haltonurology.com What is a hydrocelectomy, spermatocelectomy and epididymal cystectomy? A hydrocele is an abnormal fluid collection between the outer tissue layers of the testicle. These tissue layers naturally secrete fluid and when this fluid is not reabsorbed, as it usually would be, a fluid collection or hydrocele forms. The cause of most hydroceles is unknown, although some may be related to trauma, infection, or past surgery. A spermatocele is a cyst-like sac that is usually attached to the epididymis, the tube that sits behind the testicle and stores sperm. The sac of a spermatocele is filled with sperm. The exact cause of a spermatocele is unknown but it is thought that injury and obstruction may play a part in their formation. An epididymal cyst is much the same as a spermatocele. However, the sac attached to the epididymis is a true cyst and is filled with cystic fluid and not sperm. A hydrocelectomy is an operation to treat a hydrocele. An incision is made in the scrotum and the testicle containing the hydrocele is lifted out. The sac is then removed and the remaining tissue edges are stitched back. The tissue edges then heal onto themselves and the surrounding vessels naturally reabsorb any fluid produced. -

Hemospermia: Long-Term Outcome in 165 Patients
International Journal of Impotence Research (2013) 26, 83–86 & 2013 Macmillan Publishers Limited All rights reserved 0955-9930/13 www.nature.com/ijir ORIGINAL ARTICLE Hemospermia: long-term outcome in 165 patients J Zargooshi, S Nourizad, S Vaziri, MR Nikbakht, A Almasi, K Ghadiri, S Bidhendi, H Khazaie, H Motaee, S Malek-Khosravi, N Farshchian, M Rezaei, Z Rahimi, R Khalili, L Yazdaani, K Najafinia and M Hatam Long-term course of hemospermia has not been addressed in the sexual medicine literature. We report our 15 years’ experience. From 1997 to 2012, 165 patients presented with hemospermia. Mean age was 38 years. Mean follow-up was 83 months. Laboratory evaluation and testis and transabdominal ultrasonography was done in all. Since 2008, all sonographies were done by the first author. One patient had urinary tuberculosis, one had bladder tumor and three had benign lesions at verumontanum. One patient had bilateral partial ejaculatory duct obstruction by stones. All six patients had persistent, frequently recurring or high-volume hemospermia. All pathologies were found in young patients. In the remaining 159 patients (96%), empiric treatment was given with a fluoroquinolone (Ciprofloxacin) plus an nonsteroidal anti-inflammatory drug (Celecoxib). In our 15 years of follow-up, no patient later developed life-threatening disease. Diagnostic evaluation of hemospermia is not worthwhile in the absolute majority of cases. Advanced age makes no difference. Only high-risk patients need to be evaluated. The vast majority of cases may be safely and -

Acute Scrotum Medical Student Curriculum
ADVERTISEMENT 0 AUAU My AUA Join Journal of Urology Guidelines Annual Meeting 2017 ABOUT US EDUCATION RESEARCH ADVOCACY INTERNATIONAL PRACTICE RESOURCES Are you a Patient? EDUCATION > Educational Programs > Education for Medical Students > Medical Student Curriculum > Acute Scrotum Medical Student Curriculum ACUTE SCROTUM This document was amended in July 2016 to reflect literature that was released since the original publication of this content in May 2012. This document will continue to be periodically updated to reflect the growing body of literature related to this topic. KEY WORDS: Testis, epididymis, torsion, epididymitis, ischemia, tumor, infection, hernia Learning Objectives ADVERTISEMENT At the end of medical school, the student should be able to: ADVERTISEMENT 1. Describe 6 conditions that may produce acute scrotal pain or swelling. 2. Distinguish, through the history, physical examination and laboratory testing, testicular torsion, torsion of testicular appendices, epididymitis, testicular tumor, scrotal trauma and hernia. 3. Appropriately order imaging studies to make the diagnosis of the acute scrotum. 4. Determine which acute scrotal conditions require emergent surgery and which may be handled less emergently or electively. Introduction The "acute scrotum" may be viewed as the urologist's equivalent to the general surgeon's "acute abdomen." Both conditions are guided by similar management principles: The patient history and physical examination are key to the diagnosis and often guide decision making regarding whether or not surgical intervention is appropriate. Imaging studies should complement, but not replace, sound clinical judgment. When making a decision for conservative, non-surgical care, the provider must balance the potential morbidity of surgical exploration against the potential cost of missing a surgical diagnosis. -

Step-By-Step: Male Genital Examination Examination of Male Genitals and Secondary Sexual Characteristics
Step-by-Step: Male Genital Examination Examination of male genitals and secondary sexual characteristics. Testicular volume Testicular volume is assessed using an orchidometer; a sequential series of beads ranging from 1 mL to 35 mL (see Image 1). Testicular volume is measured using the following steps: 1. Conduct the examination in a warm environment, with the patient lying on his back 2. Gently isolate the testis and distinguish it from the epididymis. Then stretch the scrotal skin, without compressing the testis 3. Use your orchidometer to make a manual side-by-side comparison between the testis and beads (see image 2) 4. Identify the bead most similar in size to the testis, while making allowance not to include the scrotal skin. Normal testicular volume ranges Childhood Puberty Adulthood Image 1 – Orchidometer < 3 mL 4-14 mL 15-35 mL Why use an orchidometer? Clinical notes Testicular volume is important in the diagnosis of androgen • Asymmetry between testes is common (e.g. 15 mL versus defi ciency, infertility and Klinefelter syndrome. 20 mL) and not medically signifi cant • Asymmetry is sometimes more marked following unilateral testicular damage • Testes are roughly proportional to body size • Reduced testicular volume suggests impaired spermatogenesis • Small testes (<4 mL) from mid puberty are a consistent feature of Klinefelter syndrome Examination of secondary sexual characteristics Gynecomastia • Gynecomastia is the excessive and persistent development of benign glandular tissue evenly distributed in a sub-areolar position of -

Guidelines on Chronic Pelvic Pain
European Association of Urology GUIDELINES ON CHRONIC PELVIC PAIN M. Fall (chair), A.P. Baranowski, C.J. Fowler, V. Lepinard, J.G.Malone-Lee, E.J. Messelink, F. Oberpenning, J.L. Osborne, S. Schumacher. FEBRUARY 2003 TABLE OF CONTENTS PAGE 5 CHRONIC PELVIC PAIN 5.1 Background 4 5.1.1 Introduction 4 5.2 Definitions of chronic pelvic pain and terminology 4 5.3 Classification of chronic pelvic pain syndromes 6 Appendix - IASP classification as relevant to chronic pelvic pain 7 ` 5.4 References 8 5.5 Chronic prostatitis 8 5.5.1 Introduction 8 5.5.2 Definition 8 5.5.3 Pathogenesis 8 5.5.4 Diagnosis 9 5.5.5 Treatment 9 5.6 Interstitial Cystitis 10 5.6.1 Introduction 10 5.6.2 Definition 10 5.6.3 Pathogenesis 11 5.6.4 Epidemiology 12 5.6.5 Association with other diseases 13 5.6.6 Diagnosis 13 5.6.7 IC in children and males 13 5.6.8 Medical treatment 14 5.6.9 Intravesical treatment 15 5.6.10 Interventional treatments 16 5.6.11 Alternative and complementary treatments 17 5.6.12 Surgical treatment 18 5.7 Scrotal Pain 22 5.7.1 Introduction 22 5.7.2 Innervation of the scrotum and the scrotal contents 22 5.7.3 Clinical examination 22 5.7.4 Differential Diagnoses 22 5.7.5 Treatment 23 5.8 Urethral syndrome 23 5.9 References 24 6. PELVIC PAIN IN GYNAECOLOGICAL PRACTICE 36 6.1 Introduction 36 6.2 Clinical history 36 6.3 Clinical examination 36 6.3.1 Investigations 36 6.4 Dysmenorrhoea 36 6.5 Infection 37 6.5.1 Treatment 37 6.6 Endometriosis 37 6.6.1 Treatment 37 6.7 Gynaecological malignancy 37 6.8 Injuries related to childbirth 37 6.9 Conclusion 38 6.10 References 38 7. -

Outcomes and Complications in Surgical and Urological Procedures
Outcomes and complications in surgical and urological procedures Karl-Johan Lundström Institutionen för kirurgisk och perioperativa vetenskaper Umeå 2017 Responsible publisher under swedish law: the Dean of the Medical Faculty This work is protected by the Swedish Copyright Legislation (Act 1960:729) ISBN: 978-91-7601-717-3 ISSN: 0346-6612 1HZ6HULHV1R1899 Omslagsbild: Anna Erlandsson Elektronisk version tillgänglig på http://umu.diva-portal.org/ Tryck/Printed by: UmU-tryckservice, Umeå universitet Umeå, Sverige 2017 If it ain´t broke, why fix it? - Ronnie Coleman Table of Contents i Abstract ii List of papers iiv Använda förkortningar v Enkel sammanfattning på svenska vi Bakgrund vi Mål vi Metod vi Resultat vii Slutsatser vii Introduction Overveiw 1 Groin hernia 4 Hydrocele 7 Prostate biopsy 10 Complications 11 Methodological considerations 14 Registries used in this thesis 15 Materials and methods Aims of this thesis 16 Patients, materials and methods Paper I 17 PaperII 18 Paper III 21 Paper IV 22 Paper V 27 Statistics 28 Results Paper I 29 Paper II 31 Paper III 33 Paper IV 34 Paper V 38 Discussion General 40 Paper I and II 45 Paper III amd IV 47 Paper V 49 Strenghts and weaknesses 51 Conclusions 52 Future perspectives 53 Acknowledgements 54 References 56 i Abstract Background: Minor procedures in surgery and urology such as groin hernia and hydrocele repair, as well as prostate biopsies are very frequent in routine cinical practice. Complications and poor outcomes that affect many patients with a significant cumulative effect is of major importance from a population perspective. Nevertheless, there is poos scientific evidence behind som of these procedures. -

Editorial for Andrology Open Access
ISSN: 2167-0250 Andrology Open Access Editorial A Retrospective Study of Metabolic Memory Effects in Diabetic Men Evelyn K* Longdom Group SA, Avenue Roger Vandendriessche, Brussels, Belgium Editorial In the 2 issues of Volume 8 published during the year 2019, a total of 3 articles were published (at an average of 1 article per I am pleased to introduce Andrology Open Access (ANO) a issue) of which, articles were published from authors all around rapid peer-reviewed Journal which has a medical specialty that the world. A total of 10 research scientists from all over the deals with male health, particularly related to the male world reviewed the 3 articles published in volume 8. The average reproductive system and urological syndromes by exploring the publication period of an article was further reduced to 14-21 Azoospermia, Cryptorchidism, Frenulum Breve, Hypospadias, days. Low Libido, Penile cancer, Penile Fracture, Phimosis, Priapism, Retrograde Ejaculation, Semen Analysis, Spermatocele, Andrology Open Access Journal also announces its new Testicular Cancer, Testosterone, Varicocele, Vasectomy. I am association with Longdom Group for Archiving, Journal pleased to announce that, all issues of volume 8 were published maintenance, financial purpose, and support. However, the online well within the time, and the print issues were also journal will be running its original website https:// brought out and dispatched within 30 days of publishing the www.longdom.org/andrology-open-access.html parallel for issue online during the year of 2019. Editorial and review work process to maintain its highest standard of scientific work. The Journals aim to flourish and to maintain the standards in research and practice, provide platform and opportunity to During the year 2019, a total of One Editors, One Reviewers present evidence-based Andrology and medical assessment of joined the board of ANO and contributed their valuable services research, and probably it is much indeed for students, teachers, towards contribution as well as the publication of articles, and and professors. -
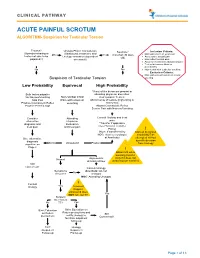
ACUTE PAINFUL SCROTUM ALGORITHM- Suspicion for Testicular Torsion
CLINICAL PATHWAY ACUTE PAINFUL SCROTUM ALGORITHM- Suspicion for Testicular Torsion Trauma? Urology Phone Consultation Neonate? Inclusion Criteria: (Open/penetrating or (Ultrasound, treatment, and Yes Yes (less than 30 days • Male patients 0-21 years old testes not able to be Urology evaluation dependent • Acute onset scrotal pain old) palpated?) on consult) • Intermittent scrotal pain • Acute or intermittent abdominal pain • Testicular trauma: blunt or penetrating No No • Non-verbal with testicular swelling Exclusion Criteria: • Male patients with painless scrotal Suspicion of Testicular Torsion swelling Low Probability Equivocal High Probability *If any of the below are present or Both testes palpable attending physician discretion: No Nausea/Vomiting Non-Verbal Child Can’t palpate Testicle Mild Pain Pain with nausea/ Abnormal lie of testicle (high-riding or Positive Cremasteric Reflex vomiting horizontal) Positive Prehn’s Sign Absent Cremasteric Reflex Severe Pain with Nausea/Vomiting Consider Attending Consult Urology and treat alternative Physician pain diagnosis and Evaluation *Transfer if applicable treat pain and treat pain (see EMTALA Transfer Policy) ! (Note: If transferred by Manual Detorsion NOC, must see urologist should NOT be See alternative at Anschutz) attempted without diagnosis specific direction Alt Dx Ultrasound Positive algorithm on from Urology Page 2 ! Obtain US while awaiting transfer (only if it does not Normal Asymmetric diminished flow delay/impede transfer) Still concerned? Consult Urology Symptoms Anschutz: -

Significance of Testicular Microlithiasis
Urol Int 2005;75:3–7 DOI: 10.1159/000085918 Review Stefan Zastrow Oliver W. Hakenberg Signifi cance of Testicular Manfred P. Wirth Microlithiasis Department of Urology, University Hospital ‘Carl Gustav Carus’, Technical University of Dresden , Dresden, Germany Abstract healthy individuals remains unclear, because most stud- ies have been done in patients who presented with scrotal Introduction: Testicular microlithiasis is an uncom- symptoms. The aim of this article is to review the current mon condition characterized by calcifi cations within the knowledge about this entity. seminiferous tubules. The true prevalence in a normal population has not been defi ned. Methods: A review of the literature with emphasis on the connection between Morphology testicular microlithiasis and testicular malignancy was carried out. Results: Testicular microlithiasis is associ- Morphologically, the microliths consist of degenerated ated with different testicular pathologies, including tes- intratubular cells which form a calcifi ed core. This core ticular cancer. However, a direct causative connection is surrounded by a series of concentric layers. The outer between testicular microlithiasis and testicular patholo- gies is not supported by the literature. Conclusions: Pa- tients with testicular microlithiasis should be followed up regularly. Further investigations concerning the etiol- Fig. 1. Testicular microlithiasis in a 23 year old male presenting ogy of testicular microlithiasis remain to be done. with scrotal pain. Copyright © 2005 S. Karger AG, Basel Introduction Testicular microlithiasis is a condition characterized by multiple calcifi cations distributed randomly through- out the testicular parenchyma (fi g.1). It was fi rst described radiologically by Priebe and Garret [1] in 1970. The ap- pearance on ultrasound is typical with scattered multiple echogenic lesions distributed over the testis. -

Pediatric Urology Guidelines for Central Scheduling
Pediatric Urology Guidelines for Central Scheduling *Designed to reduce risk of infection, organ injury or loss, misdiagnosis of possible malignancy, mental anquish for patients and families, while providing optimal medical care and stewardship SYSTEM DIAGNOSES COLOR CODE KEY TESTIS/SCROTUM Column1 VOIDING ISSUES Column1 OFFICE APPOINTMENTS* Absent testis Bedwetting/Nocturnal Enuresis ER Emergent - send to ER Epididymitis/Orchitis Bladder diverticula OFFICE Urgent (within 1 week) Hydrocele Bladder exstrophy OFFICE Semi-Urgent (within 3 weeks) Hernia/Inguinal/Umbilical Bladder mass/tumor OFFICE Elective (deferred after 3 months) - need Retractile testis Blood in urine/Hematuria to track so we can schedule Undescended testis/UDT Dysuria Spermatocele/Epididymal cyst Incontinence/Daytime Enuresis/Leaking TELEMEDICINE APPOINTMENTS* Testis/Scrotum Mass Hematuria - microscopic TELEMEDICINE - urgent (within 24hrs) Varicocele Hematuria - gross TELEMEDICINE - semi-urgent (within 3 weeks) Testis pain/torsion Frequency/Polyuria/Urgency TELEMEDICINE - non-urgent (can be after 3 Ureterocele weeks) BLADDER Column1 Acute Cystitis/UTI KIDNEY ISSUES Neurogenic bladder/Spina Bifida- established Elevated creatinine /Renal failure Urachus Cystic kidney disease/MCDK/cysts Prune Belly Syndrome Duplex collecting system Acute urinary retention Ectopic kidney Horseshoe kidney URETHRA Column1 Hydronephrosis/Pyelectasis Urethral prolapse Hydroureter/Megaureter Urethral stricture Stones - Symptomatic-Pain Stones - Asymptomatic-No pain PENIS Column1 Prenatal/Antenatal -

Do I Have Testicular Cancer?
Do I Have Testicular Cancer? Men who notice lumps, swelling, or pain in their groin or scrotum may worry they have testicular cancer. Here we describe the symptoms of testicular cancer and some other problems that could cause symptoms in this part of the body. We also include information on how to do a testicular self-exam for men who want to do so. This is not meant to be a complete guide to testicular symptoms, nor is it meant to give medical advice or replace the expertise and judgment of a health care provider. If you notice any changes in your testicles, you should see a provider so that the cause can be found and treated, if needed. The testicles Testicles are a part of the male reproductive system. In adult males, these 2 organs are each normally a little smaller than a golf ball. They are contained within a sac of skin called the scrotum, which hangs beneath the base of the penis. Testicles have 2 main functions: ● They make male hormones, like testosterone. ● They make sperm, the male cells needed to fertilize a female’s egg to start a pregnancy. Sperm cells form inside the testicle and are then stored in the epididymis (EP-ih-DID- uh-mus), a small coiled tube behind each testicle, where they mature. When a man ejaculates ( has an orgasm), sperm cells travel from the epididymis through the vas deferens (vass DEF-er-ens) to the seminal vesicles (SIM-uh-nul VES- ih-kuls), where they mix with fluids made by the vesicles, the prostate gland, and other glands to form semen. -

Overview of Urology Clinical Services
Overview of Urology Clinical Services Jerilyn M. Latini, M.D. Service Center Medical Director Department of Urology Alaska Native Medical Center Alaska Native Tribal Health Consortium August 2014 Urology Services Our Team Includes: • 3 Urologists (MDs) – adult urology • 2 Urologists (MDs) – pediatric urology (consultants) • 2 Urology Physician Assistants (PAs) We deliver patient care via: • In-person clinic visits at ANMC • In-person field clinic visits • Kotzebue • Nome • Bethel • Dillingham • Ketchikan • SEARHC Sitka • SEARHC Juneau • Electronic consultations (AFHCAN) Urology Services We provide an array of surgical services for patients of all ages including: • Benign & malignant conditions affecting the urinary system • Benign & malignant conditions affecting the male reproductive system • Urologists perform surgery on the kidneys, ureters, bladder, urethra in males & females • Urologists perform surgery on the penis, testes, epididymes, prostate in males ** Benign & malignant conditions affecting the female reproductive system, as well as female urinary incontinence and pelvic organ prolapse, are managed by Women’s Health / Obstetrics & Gynecology ** Urology Services We evaluate & provide surgical management of urologic conditions for pediatric patients including: • Benign conditions affecting the urinary system in girls & boys • Urinary tract infections • Reflux of urine • Urinary symptoms & difficulty urinating • Incontinence (leakage of urine) and Enuresis (bedwetting) • Urinary stones (calculi) • Benign conditions affecting the