Original Paper Comparison of Volatile Compounds in 'Fuji' Apples in The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phase Change Indicators for Subambient Temperatures
II NASA CONTRACTOR REPORT 40 h ,-. ..../_,.,_..,.. - . ;:" ;'; _...,: -I e U PHASE CHANGE INDICATORS FOR SUBAMBIENT TEMPERATURES by D. R. Kasanof and E. Kimmel Prepared by i TEMPILO CORPORATION New York, N. Y. for LatzgZey Research Center NATIONALAERONAUTICS AND SPACE ADMINISTRATION WASHINGTON, D. C. MARCH 1970 ._ TECH LIBRARY KAFB, NM 00b0899 c-w, J NASA CR-1576 /PHASE CHANGE INDICATORS FOR SUBAMBIENT TEMPERATURES bL&~Q- By D. R. Kasanof and E. Kimmel Distribution of this report is provided in the interest of informationexchange. Responsibility for thecontents resides in the author or organization that prepared it. \ p- ' '\.prepared under ContractNO. L/.@&J31.-566by Luc.g9 "-.._--. TEMPILO CORPORATION New York, N.Y. for Langley Research Center NATIONAL AERONAUTICS AND SPACE ADMINISTRATION For sale by the Clearinghouse for Federal Scientific ond Technical Information Springfield,Virginia 22151 - CFSTI price $3.00 PHASE CIIANGE INDICATORS FOR SUBAMBIENTTEMPERATURES By D. R. Kasanof and E. Kinnnel TEMPIL" CORPORATION Forty candidate organic compounds were evaluated as possible fusible temperature indicators for use in the range -50°F to -150°F. Of these, thirty with melting points ranging from -37°F to -162°F were found to be useful as temperature indicators. Nine candidates were rejected because they did not yield opaque, crystalline coatings by any of the application procedures attempted. One candidate material was rejected because it proved to be unstable. Phase studies of several binary systems were also carried out. A number of application procedures were evaluated. An aerosol-spray procedure was adopted because it consistently yielded opaque coatings, was relatively simple to employ and could probably be adapted for use in the Langley Field test apparatus. -

Interagency Committee on Chemical Management
DECEMBER 14, 2018 INTERAGENCY COMMITTEE ON CHEMICAL MANAGEMENT EXECUTIVE ORDER NO. 13-17 REPORT TO THE GOVERNOR WALKE, PETER Table of Contents Executive Summary ...................................................................................................................... 2 I. Introduction .......................................................................................................................... 3 II. Recommended Statutory Amendments or Regulatory Changes to Existing Recordkeeping and Reporting Requirements that are Required to Facilitate Assessment of Risks to Human Health and the Environment Posed by Chemical Use in the State ............................................................................................................................ 5 III. Summary of Chemical Use in the State Based on Reported Chemical Inventories....... 8 IV. Summary of Identified Risks to Human Health and the Environment from Reported Chemical Inventories ........................................................................................................... 9 V. Summary of any change under Federal Statute or Rule affecting the Regulation of Chemicals in the State ....................................................................................................... 12 VI. Recommended Legislative or Regulatory Action to Reduce Risks to Human Health and the Environment from Regulated and Unregulated Chemicals of Emerging Concern .............................................................................................................................. -

Dependent Modeling Approach Derived from Semi-Empirical Quantum Mechanical Calculations
3D-QSAR/QSPR Based Surface- Dependent Modeling Approach Derived From Semi-Empirical Quantum Mechanical Calculations 3D-QSAR/QSPR-basierter, oberflächenabhängiger Modellierungsansatz, abgeleitet von semi-empirischen quantenmechanischen Rechnungen Der Naturwissenschaftlichen Fakultät der Friedrich-Alexander-Universität Erlangen-Nürnberg Zur Erlangung des Doktorgrades Dr. rer. nat. vorgelegt von Marcel Youmbi Foka aus Kamerun Als Dissertation genehmigt von der Naturwissenschaftlichen Fakultät/ vom Fachbereich Chemie und Pharmazie der Friedrich-Alexander-Universität Erlangen-Nürnberg Tag der mündlichen Prüfung: 05.12.2018 Vorsitzender des Promotionsorgans: Prof. Dr. Georg Kreimer Gutachter/in: Prof. Dr. Tim Clark Prof. Dr. Birgit Strodel Dedication In memory of my late Mother Lucienne Metiegam, who the Lord has taken unto himself on May 3, 2009. My mother, light of my life, God rest her soul, had a special respect for my studies. She had always encouraged me to move forward. I sincerely regret the fact that today she cannot witness the culmination of this work. Maman, que la Terre de nos Ancêtres te soit légère! This is a special reward for Mr. Joseph Tchokoanssi Ngouanbe, who always supported me financially and morally. That he find here the expression of my deep gratitude. i ii Acknowledgements I would like to pay tribute to all those who have made any contribution, whether scientific or not, to help carry out this work. All my thanks go especially to Prof. Dr. Tim Clark, who gave me the opportunity and means to work in his research team. I am grateful to have had him not only supervise my work but also for his patience and for giving me the opportunity to explore this fascinating topic. -

Laboratory 24: Properties of Carboxylic Acids and Esters Introduction Discussion
Laboratory 24: Properties of Carboxylic Acids and Esters Introduction In this laboratory we will explore the chemical and physical properties of carboxylic acids and esters. Discussion Structure of Carboxylic Acids and Esters Aldehydes and Ketones are organic compounds containing a carbonyl carbon ( R−COO – ) (Figure 1 below) connected with an oxygen atom. The carbonyl carbon is a polar group with the carbon having a slight excess of positive charge and the oxygen atom having a slight excess of negative charge. The addition of a second oxygen atom increases the strength of the dipole. Both molecules are capable of dipole-dipole interactions. One major difference between the molecules is the hydrogen atom attached to the oxygen atom in carboxylic acids which allows for the formation of hydrogen bonds between molecules. Carboxylic acids are often responsible for many flavors such as the tart taste of vinegar which is commonly called acetic acid. The sour taste of fruits such as lemons is due to the presence of citric acid. Unlike carboxylic acids which often have tart tastes or unpleasant odors, many esters have pleasant flavors and fragrances. The odor and flavor of oranges is due to octyl propanoate, that of pears due to pentyl propanoate, and raspberries from 2-methylpropyl methanoate. Chemical Properties Carboxylic acids are considered weak acids because the carboxyl group ionizes slightly in water. + )−−−−* + Carboxylic acids can react with bases to form carboxylic acid salts. Large carboxylic acids are insoluble in water, however, conversion to a salt greatly increases the solubility in water. Many carboxylic acids found in food products or medications are found in their soluable salt form rather than as the acid itself. -

합성향료 Synthetic Flavoring Substances 정 <식약처 고시 제2021
<식약처 고시 제2021-00호, 2021.6.00.> [시행일 2022.7.1.] ※ 합성향료를 삭제하고, 천연향료와 통합하여 향료로 신설 합성향료 Synthetic Flavoring Substances 정 의 식품에 착향의 목적으로 사용할 수 있는 합성향료는 아래와 같다. 다만, 이 품목에는 아래의 향료를 화학적 변화를 주지 않는 방법으로 2종 이상 단순 혼합한 것이 포함되며, 품질보존, 희석, 용해, 분산 등을 위하여 물, 주정, 프로필렌글리콜, 트리아세틴, 글리세린, 덱스트린, 전분, 변성전분, 아라비아검, 이산화규소를 첨가할 수 있다. 순 번 일 반 명 이 명 Ethylidine diethyl acetal; acetaldehyde diethyl acetal; diethyl acetal; A001 Acetal 1,1-Diethoxyethane; Ethylidine diethyl ether; 1,1-Diethoxyethane A002 Acetaldehyde Acetic aldehyde; Ethanal; Ethyl aldehyde Acetaldehyde butyl phenethyl 2-Butoxy-2-phenylethoxy-ethane; A003 acetal 1-Butoxy-1-(2-phenylethoxy)ethane Acetaldehyde diisoamyl Butane, 1,1'-[ethylidenebis(oxy)]bis[3-methyl]-, A004 acetal 3-Methyl-1-[1-(3-methyl-butoxy)-ethoxy]-butane Ethyl cis-3-hexenyl acetal; cis-1-(ethoxyethoxy)-3-hexene; Acetaldehyde ethyl A005 1-Ethoxy-1-(cis-3-hexenyloxy)ethane, Leaf acetal; Leaf cis-3-hexenyl acetal alcohol ethyl acetal; Acetaldehyde ethyl (z)-3-hexenyl acetal; - 1 - 1-Ethoxy-1-(3-hexenyloxy)ethane; Acetaldehyde ethyl 3-hexenyl acetal Benzene, 2-(1-propoxyethoxy)ethyl; Acetal R; pepital; Acetaldehyde phenethyl 1-Phenethoxy-1-propexy-ethane; A006 propyl acetal Propyl phenethyl acetal; 2-(1-Propoxyethoxy)ethyl]benzene Acetic acid amide; Acetimidic acid; Ethanamide; A007 Acetamide Ethanamidic acid; Methanecarboxamide Methyl 4-methoxyphenyl ketone; 4-Acetylanisole; p-Acetyl anisole; A008 Acetanisole p-Methoxy-acetophenone; Navatone; 1-(4-Methoxyphenyl)ethanone; 4-Methoxyacetophenone Acetyl methyl carbinol; 2,3-Butanolone; Dimethylketol; -
ECSA Chemicals Catalogue – Flavours and Fragrances
FLAVOURS & FRAGRANCES CATALOGUE The ECSA Group is a fourth generation family firm. The Group includes theECSA Chemicals, ECSA Maintenance and ECSA Energy with headquarters based in Balerna (Ticino) and Flawil (St. Gallen), the sister company ECSA Italia based in Desio (Milan) and since 2012 Porta Ticino Easy Stop SA. FLAWIL BALERNA DESIO ECSA is today the largest Swiss owned company in the chemical distribution, specialising in following segments: Cosmetics, Pharmaceuticals, Food, Non-Essential Products & Feed, Flavours and Fragrances, Metal treatment, Water treatment, Detergents, Reagents, Paints feedstock, Textiles and Leather, Rubber and Plastics, Base chemicals. 2 FLAVOURS & FRAGRANCES INDEX Essential Oils ....................................................................................4 Balsams & Resinoides .....................................................................5 Natural Isolates ................................................................................6 Aroma Chemicals .............................................................................7 Food Ingredients .............................................................................19 Cosmetic Ingredients .....................................................................20 Alphabetical Index ..........................................................................21 CAS Index ........................................................................................24 CATALOGUE 3 ESSENTIAL OILS Product Botanical source CAS FEMA Anis Star Illicium verum -

Cold-Storage Potential of Four Yellow-Fleshed Peach Cultivars
Article pubs.acs.org/JAFC Cold-Storage Potential of Four Yellow-Fleshed Peach Cultivars Defined by Their Volatile Compounds Emissions, Standard Quality Parameters, and Consumer Acceptance † † § ,† Jaime Cano-Salazar, Gemma Echeverría, Carlos H. Crisosto, and Luisa Lopez* † Àrea de Post-Collita, XaRTA, UdL-IRTA, Alcalde Rovira Roure 191, 25198 Lleida, Spain § Department of Plant Sciences, University of California, One Shields Avenue, Davis, California 95616, United States ABSTRACT: ‘Early Rich’, ‘Royal Glory’, ‘Sweet Dreamcov’, and ‘Elegant Lady’ peaches were stored at −0.5 °C for up to 40 days and then subjected to ripening at 20 °C for up to 3 days. Firmness, soluble solids content (SSC), titratable acidity (TA), color, consumer acceptance, and volatile compounds were then determined. The observed physicochemical changes included a significant decrease in firmness during both storage and commercialization periods. In contrast, the SSC, TA, and color remained constant during storage. Ten days of cold storage produced the highest total volatile emissions and the greatest consumer acceptance for ‘Elegant Lady’ and ‘Sweet Dreamcov’, whereas similar results were obtained after 40 and 20 days for ‘Royal Glory’ and ‘Early Rich’, respectively. Volatile compounds that most consistently exhibited a positive correlation with consumer acceptance were dependent on the cultivar. KEYWORDS: cold storage, consumer acceptance, peach, partial least-squares regression model, volatile compounds ■ INTRODUCTION In this cultivar, the presence of γ-decalactone, δ-octalactone, Peach (Prunus persica L. Batsch) is a climacteric stone fruit γ-octalactone, ethyl butyrate, hexanal, and (E)-2-hexenol is a 9 species that provides high nutrition and a pleasant flavor.1 good indicator of maturity at harvest. -

Use of High Throughput Assays and Computational Tools; Endocrine Disruptor Screening Program; Notice of Availability and Opportunity for Comment’’
NRDC EPA-HQ-OPPT-2015-0305 August, 2015 Comments from the Natural Resources Defense Council On The Document Titled, ‘‘Use of High Throughput Assays and Computational Tools; Endocrine Disruptor Screening Program; Notice of Availability and Opportunity for Comment’’ To the U.S. Environmental Protection Agency Docket No. EPA-HQ-OPPT-2015-0305 August 18, 2015 Background The Natural Resources Defense Council ("NRDC") is a national, non-profit environmental organization of lawyers, scientists, and other professionals. NRDC presents these comments on behalf of our 1.4 million members and online activists. NRDC does not have any financial interest in the topic of these comments. The endocrine system utilizes highly complex, tightly controlled molecular processes for its optimal functioning in the body. The proper balance of hormones can be synchronized in a variety of ways (including direct protein binding, epigenetic alterations, gene activation and silencing), and is essential across the entirety of the life course. Small changes in the perfectly orchestrated symphony of hormone levels can severely disrupt the harmony necessary for critical windows of development (e.g., fetal development, infanthood, childhood, and adolescence), leaving the body vulnerable to a host of negative health outcomes (such as diabetes, cancer, obesity, and reproductive dysfunction). The last decade has seen an exponential increase in the development of computational, biological, and chemical tools capable of increasing both the number of chemicals analyzed and the pace of chemical toxicity evaluation. These tools, including the EPA Toxicity Forecaster (ToxCast™) and the National Institute of Environmental Health Sciences (NIEHS) Tox21 platforms, have the potential to rapidly generate molecular and cellular data for thousands of chemicals at once, and provide an additional stream of useful information that can aide in regulatory decision-making. -

Changes in DNA the Fruit Ripening-Related Gene Faaat2
Journal of Experimental Botany , Vol. Page 63, 63, 1 ofNo. No. 16 11, 2, pp. pp. 695–709, 4275–4290, 2012 2012 doi:10.1093/jxb/err313doi:10.1093/jxb/ers120 AdvanceAdvance AccessAccess publicationpublication 44 November,May, 2012 2011 This paper is available online free of all access charges (see http://jxb.oxfordjournals.org/open_access.html for further details) RESEARCH PAPER Journal of Experimental Botany 63 InThePosidonia fruit ripening-related oceanica cadmium gene FaAAT2 inducesencodes changes an in acyl DNA 11 4275 methylationtransferase involved and chromatin in strawberry patterning aroma biogenesis 4290 2012 MariaGuadalupe Greco, Cumplido-Laso Adriana Chiappetta,1, Laura Leonardo Medina-Puche Bruno1, and Enriqueta Maria Beatrice Moyano1 Bitonti*, Thomas Hoffmann2, Quirin Sinz2, 2 2 1 2 1 doi:10.1093/jxb/ers120 DepartmentLudwig Ring of Ecology,, Claudia University Studart-Wittkowski of Calabria, Laboratory, Jose of´ PlantLuis Cyto-physiology, Caballero , Wilfried Ponte Pietro Schwab Bucci,,JuanMun I-87036 Arcavacata˜ oz-Blanco di Rende, Advance Access publication 4 May, 2012 Cosenza,and Rosario Italy Blanco-Portales1,* Downloaded from https://academic.oup.com/jxb/article/63/11/4275/603789 by guest on 30 September 2021 Cumplido-Laso et al. *1 ToDepartamento whom correspondence de Bioquı´mica should y Biologı be addressed.´a Molecular. E-mail: Edificio [email protected] C-6, Campus Universitario de Rabanales y Campus de Excelencia Strawberry FaAAT2 is involved in fruit aroma biogenesis Internacional Agroalimentario CEIA3, Universidad de Co´ rdoba, 14071 Co´ rdoba, Spain 2 ReceivedBiotechnology 29 May of2011; Natural Revised Products, 8 July 2011; Technische Accepted Universita 18 August¨ t Mu¨ 2011nchen, Liesel-Beckmann-Str. -

1192 Date 9 October 2019 to Whom It May Concern
Subject Introduction to the outcomes of MEPC 74 Technical Information No. TEC-1192 Date 9 October 2019 To whom it may concern The seventy-fourth session of the Marine Environment Protection Committee (MEPC 74) was held at the IMO in London, U.K. from 13 to 17 May. Since the minutes, resolutions and circulars of the meeting were recently released from the IMO secretaries, a summary of the decisions taken at MEPC 74 is provided as below for your information. 1. Greenhouse Gases (GHG) emission reduction measures GHG emissions from international shipping have been deliberated at IMO, and so far, the Energy Efficiency Design Index (EEDI), the Ship Energy Efficiency Management Plan (SEEMP) and the Data Collection System for fuel oil consumption of ships (DCS) were introduced. Further, at MEPC 72, Initial IMO Strategy on reduction of GHG emissions from ships, which includes emission reduction target and candidate measures to reduce GHG emissions, was adopted. (1) Review of technological developments for EEDI Regulation 21.6 of MARPOL Annex VI sets out that a review of the status of technological developments which may contribute to the improvement of EEDI should be conducted. It also requires, if proved necessary, to amend the subsequent requirements, i.e. "when to start the each phase" and "the reduction rate". At MEPC 71, it was agreed to establish a correspondence group (CG), coordinated by Japan, to consider an early implementation of phase 3 and possible introduction of phase 4. At this session, consideration was made based on the report of the CG and agreements reached at MEPC 73. -
PDF-Document
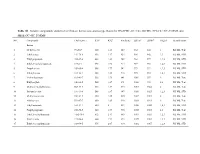
Table S1. Volatile compounds identified in Chinese herbaceous aroma-type Baijiu by HS-SPME-GC×GC-TOFMS, SPE-GC×GC-TOFMS and SBSE-GC×GC-TOFMS. NO. Compounds CAS number RT1a RT2b Similarity LRIcalc LRIlitd Origine Identificationf Esters 1 Methyl acetate 79-20-9 360 1.43 847 832 844 1 RI, MS, Tent 2 Ethyl acetate 141-78-6 416 1.57 924 904 902 1,3 RI, MS, STD 3 Ethyl propanoate 105-37-3 488 1.81 949 963 977 1,2,3 RI, MS, STD 4 Ethyl 2-methyl propanoate 97-62-1 496 1.95 914 969 961 1,2,3 RI, MS, STD 5 Propyl acetate 109-60-4 508 1.77 942 979 992 1,2,3 RI, MS, STD 6 2-Butyl acetate 105-46-4 528 1.85 918 995 985 1,2,3 RI, MS, STD 7 Methyl butanoate 623-42-7 536 1.73 841 1001 997 2 RI, MS, Tent 8 Ethyl acrylate 140-88-5 544 1.67 891 1006 992 2,3 RI, MS, Tent 9 Methyl 2-methylbutyrate 868-57-5 564 1.87 875 1017 1022 2 RI, MS, Tent 10 Isobutyl acetate 110-19-0 568 1.87 947 1020 1025 1,2,3 RI, MS, STD 11 Methyl isovalerate 556-24-1 580 1.85 890 1027 1011 2 RI, MS, Tent 12 Allyl acetate 591-87-7 600 1.63 920 1038 1023 2 RI, MS, Tent 13 Ethyl butanoate 105-54-4 620 2 897 1050 1025 1,2,3 RI, MS, STD 14 Propyl propionate 106-36-5 620 1.99 960 1050 1065 2,3 RI, MS, Tent 15 Ethyl 2-methylbutanoate 7452-79-1 632 2.17 849 1057 1053 1,2,3 RI, MS, STD 16 Butyl acetate 123-86-4 664 1.93 892 1075 1082 1,2,3 RI, MS, STD 17 Ethyl 3-methylbutanoate 108-64-5 676 2.07 910 1082 1067 1,2,3 RI, MS, STD 18 Methyl pentanoate 624-24-8 684 1.93 816 1086 1082 1,2,3 RI, MS, STD 19 Isobutyl isobutyrate 97-85-8 688 2.35 891 1089 1092 1,2 RI, MS, Tent 20 Isobutyl propanoate -

STAFF DRAFT That May Be Revised Without Public Notice Prior to Signature
The following draft rule is a STAFF DRAFT that may be revised without public notice prior to signature. We provide this draft document solely for the convenience of interested parties. This draft is not the official rule for purposes of public comment under the Administrative Procedure Act or for compliance. When this rule is final, a signed official version will be published in the Federal Register and appear on the government printing office web site – http://www.gpoaccess.gov/fr/. * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * 3. Part 60 is amended by adding appendix J to read as follows: Appendix J to Part 60 -- How to Determine Henry’s Law Constants, Fm Values, Fr Values, and Fe Values for organic Compounds 1. Use of Appendix and General Information. This appendix has four sections. Section 2 contains the procedures for determining Henry’s law constants, fraction measured (Fm) values, fraction removed (Fr) values, and fraction emitted (Fe) values for an individual chemical. Section 3 describes how to locate certain resources. Section 4 contains five tables and thirteen forms. You should use this appendix if you need to: 1. Determine whether a chemical has a Henry’s law constant at 25o C that is less than 0.1 y/x atmosphere per mole fraction (see section 2.1). 2. Determine a fraction measured (Fm) value for a chemical (see section 2.2). 3. Subtract the concentration of a chemical from a Method 25D concentration (see section 2.3). 4. Determine the fraction removed (Fr) value for a chemical that has a Henry’s law constant at 25o C that is greater than or equal to 0.1 y/x atmosphere per STAFF DRAFT - 09/30/2005 mole fraction (see section 2.4).