Phase Change Indicators for Subambient Temperatures
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Original Paper Comparison of Volatile Compounds in 'Fuji' Apples in The
_ Food Science and Technology Research, 23 (1), 79 89, 2017 Copyright © 2017, Japanese Society for Food Science and Technology doi: 10.3136/fstr.23.79 http://www.jsfst.or.jp Original paper Comparison of Volatile Compounds in ‘Fuji’ Apples in the Different Regions in China 1 2* 3 2 2 2 Ling QIN , Qin-Ping WEI , Wen-Huai KANG , Qiang ZHANG , Jian SUN and Song-Zhong LIU 1Department of Life Science, Hebei Normal University of Science and Technology, Qinhuangdao 066004 P. R. China 2Institute of Forestry & Pomology, Beijing Academy of Agriculture & Forestry Sciences,Beijing 100093 P.R. China 3College of Bioscience and Bioengineering, Hebei University of Science and Technology, Shijiazhuang 050018 P.R. China Received May 25, 2015 ; Accepted September 28, 2016 The characteristics of the volatiles from 43 ‘Fuji’ apples representing 14 different apple production regions in China were investigated using headspace-solid phase micro-extraction (HS-SPME) combined with gas chromatography–mass spectrometry (GC-MS). The results obtained from this experiment showed that sixty- four volatile compounds were identified in ‘Fuji’ apples collected from 43 counties in China. The major volatile compounds were identified as 2-methyl butyl acetate and hexyl acetate. The composition of volatiles and their contents in ‘Fuji’ apples varied in different regions. All of the ‘Fuji’ apple samples could be classified into the following groups using a principal component analysis of the volatiles: (1) apples with high concentrations of hexyl acetate and (Z)-3-hexenyl acetate, -
ECSA Chemicals Catalogue – Flavours and Fragrances
FLAVOURS & FRAGRANCES CATALOGUE The ECSA Group is a fourth generation family firm. The Group includes theECSA Chemicals, ECSA Maintenance and ECSA Energy with headquarters based in Balerna (Ticino) and Flawil (St. Gallen), the sister company ECSA Italia based in Desio (Milan) and since 2012 Porta Ticino Easy Stop SA. FLAWIL BALERNA DESIO ECSA is today the largest Swiss owned company in the chemical distribution, specialising in following segments: Cosmetics, Pharmaceuticals, Food, Non-Essential Products & Feed, Flavours and Fragrances, Metal treatment, Water treatment, Detergents, Reagents, Paints feedstock, Textiles and Leather, Rubber and Plastics, Base chemicals. 2 FLAVOURS & FRAGRANCES INDEX Essential Oils ....................................................................................4 Balsams & Resinoides .....................................................................5 Natural Isolates ................................................................................6 Aroma Chemicals .............................................................................7 Food Ingredients .............................................................................19 Cosmetic Ingredients .....................................................................20 Alphabetical Index ..........................................................................21 CAS Index ........................................................................................24 CATALOGUE 3 ESSENTIAL OILS Product Botanical source CAS FEMA Anis Star Illicium verum -

Cold-Storage Potential of Four Yellow-Fleshed Peach Cultivars
Article pubs.acs.org/JAFC Cold-Storage Potential of Four Yellow-Fleshed Peach Cultivars Defined by Their Volatile Compounds Emissions, Standard Quality Parameters, and Consumer Acceptance † † § ,† Jaime Cano-Salazar, Gemma Echeverría, Carlos H. Crisosto, and Luisa Lopez* † Àrea de Post-Collita, XaRTA, UdL-IRTA, Alcalde Rovira Roure 191, 25198 Lleida, Spain § Department of Plant Sciences, University of California, One Shields Avenue, Davis, California 95616, United States ABSTRACT: ‘Early Rich’, ‘Royal Glory’, ‘Sweet Dreamcov’, and ‘Elegant Lady’ peaches were stored at −0.5 °C for up to 40 days and then subjected to ripening at 20 °C for up to 3 days. Firmness, soluble solids content (SSC), titratable acidity (TA), color, consumer acceptance, and volatile compounds were then determined. The observed physicochemical changes included a significant decrease in firmness during both storage and commercialization periods. In contrast, the SSC, TA, and color remained constant during storage. Ten days of cold storage produced the highest total volatile emissions and the greatest consumer acceptance for ‘Elegant Lady’ and ‘Sweet Dreamcov’, whereas similar results were obtained after 40 and 20 days for ‘Royal Glory’ and ‘Early Rich’, respectively. Volatile compounds that most consistently exhibited a positive correlation with consumer acceptance were dependent on the cultivar. KEYWORDS: cold storage, consumer acceptance, peach, partial least-squares regression model, volatile compounds ■ INTRODUCTION In this cultivar, the presence of γ-decalactone, δ-octalactone, Peach (Prunus persica L. Batsch) is a climacteric stone fruit γ-octalactone, ethyl butyrate, hexanal, and (E)-2-hexenol is a 9 species that provides high nutrition and a pleasant flavor.1 good indicator of maturity at harvest. -

Use of High Throughput Assays and Computational Tools; Endocrine Disruptor Screening Program; Notice of Availability and Opportunity for Comment’’
NRDC EPA-HQ-OPPT-2015-0305 August, 2015 Comments from the Natural Resources Defense Council On The Document Titled, ‘‘Use of High Throughput Assays and Computational Tools; Endocrine Disruptor Screening Program; Notice of Availability and Opportunity for Comment’’ To the U.S. Environmental Protection Agency Docket No. EPA-HQ-OPPT-2015-0305 August 18, 2015 Background The Natural Resources Defense Council ("NRDC") is a national, non-profit environmental organization of lawyers, scientists, and other professionals. NRDC presents these comments on behalf of our 1.4 million members and online activists. NRDC does not have any financial interest in the topic of these comments. The endocrine system utilizes highly complex, tightly controlled molecular processes for its optimal functioning in the body. The proper balance of hormones can be synchronized in a variety of ways (including direct protein binding, epigenetic alterations, gene activation and silencing), and is essential across the entirety of the life course. Small changes in the perfectly orchestrated symphony of hormone levels can severely disrupt the harmony necessary for critical windows of development (e.g., fetal development, infanthood, childhood, and adolescence), leaving the body vulnerable to a host of negative health outcomes (such as diabetes, cancer, obesity, and reproductive dysfunction). The last decade has seen an exponential increase in the development of computational, biological, and chemical tools capable of increasing both the number of chemicals analyzed and the pace of chemical toxicity evaluation. These tools, including the EPA Toxicity Forecaster (ToxCast™) and the National Institute of Environmental Health Sciences (NIEHS) Tox21 platforms, have the potential to rapidly generate molecular and cellular data for thousands of chemicals at once, and provide an additional stream of useful information that can aide in regulatory decision-making. -
PDF-Document
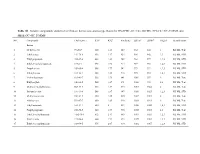
Table S1. Volatile compounds identified in Chinese herbaceous aroma-type Baijiu by HS-SPME-GC×GC-TOFMS, SPE-GC×GC-TOFMS and SBSE-GC×GC-TOFMS. NO. Compounds CAS number RT1a RT2b Similarity LRIcalc LRIlitd Origine Identificationf Esters 1 Methyl acetate 79-20-9 360 1.43 847 832 844 1 RI, MS, Tent 2 Ethyl acetate 141-78-6 416 1.57 924 904 902 1,3 RI, MS, STD 3 Ethyl propanoate 105-37-3 488 1.81 949 963 977 1,2,3 RI, MS, STD 4 Ethyl 2-methyl propanoate 97-62-1 496 1.95 914 969 961 1,2,3 RI, MS, STD 5 Propyl acetate 109-60-4 508 1.77 942 979 992 1,2,3 RI, MS, STD 6 2-Butyl acetate 105-46-4 528 1.85 918 995 985 1,2,3 RI, MS, STD 7 Methyl butanoate 623-42-7 536 1.73 841 1001 997 2 RI, MS, Tent 8 Ethyl acrylate 140-88-5 544 1.67 891 1006 992 2,3 RI, MS, Tent 9 Methyl 2-methylbutyrate 868-57-5 564 1.87 875 1017 1022 2 RI, MS, Tent 10 Isobutyl acetate 110-19-0 568 1.87 947 1020 1025 1,2,3 RI, MS, STD 11 Methyl isovalerate 556-24-1 580 1.85 890 1027 1011 2 RI, MS, Tent 12 Allyl acetate 591-87-7 600 1.63 920 1038 1023 2 RI, MS, Tent 13 Ethyl butanoate 105-54-4 620 2 897 1050 1025 1,2,3 RI, MS, STD 14 Propyl propionate 106-36-5 620 1.99 960 1050 1065 2,3 RI, MS, Tent 15 Ethyl 2-methylbutanoate 7452-79-1 632 2.17 849 1057 1053 1,2,3 RI, MS, STD 16 Butyl acetate 123-86-4 664 1.93 892 1075 1082 1,2,3 RI, MS, STD 17 Ethyl 3-methylbutanoate 108-64-5 676 2.07 910 1082 1067 1,2,3 RI, MS, STD 18 Methyl pentanoate 624-24-8 684 1.93 816 1086 1082 1,2,3 RI, MS, STD 19 Isobutyl isobutyrate 97-85-8 688 2.35 891 1089 1092 1,2 RI, MS, Tent 20 Isobutyl propanoate -

UNIVERSITY of CALIFORNIA Santa Barbara Machine Learning For
UNIVERSITY OF CALIFORNIA Santa Barbara Machine Learning for Addressing Data Deficiencies in Life Cycle Assessment A dissertation submitted in partial satisfaction of the requirements for the degree Doctor of Philosophy in Environmental Science and Management by Runsheng Song Committee in charge: Professor Sangwon Suh, Co-Chair Professor Arturo A. Keller, Co-Chair Professor Krzysztof Janowicz March 2019 The dissertation of Runsheng Song is approved. _____________________________________________ Arturo A. Keller _____________________________________________ Krzysztof Janowicz _____________________________________________ Sangwon Suh, Committee Chair March 2019 Machine Learning for Addressing Data Deficiencies in Life Cycle Assessment Copyright © 2019 by Runsheng Song iii ACKNOWLEDGEMENTS I have the privilege to work with a group of smart people during my stay at the Bren School of Environmental Science and Management, UC Santa Barbara. I would like to first give my thanks to Prof. Sangwon Suh, my PhD advisor, who provides me countless guide, and offered invaluable helps during many difficult times over my PhD career. I would like to thank Prof. Arturo A. Keller, who is always encouraging and generous when I was confused or making mistake over the development of my research. I also want to thank Prof. Krzysztof Janowicz, who shines his wisdom from another field to my PhD research, and always pointing out the room for improvement in my study which I could never figure out without him. For what I learnt from my professors, I appreciate that. I want to give thanks to my lab mates, who shared probably one of the most important periods in my career. I learnt how to be humble, kind, patient and professional working with such a smart group of people. -

2018UBFCK013.Pdf
UNIVERSITÉ DE BOURGOGNE-FRANCHE COMTÉ UNIVERSITÉ DE DIJON AGROSUP-DIJON INSTITUT NATIONAL SUPERIEUR DES SCIENCES AGRONOMIQUES, DE L’ALIMENTATION ET DE L’ENVIRONNEMENT UMR Procédés Alimentaires et Microbiologiques ÉCOLE DOCTORALE ENVIRONNEMENTS-SANTÉ THÈSE Présentée pour obtenir le grade de Docteur de l’Université de Bourgogne-Franche Comté Spécialité : Biotechnologie Agro-alimentaire Options : Biotechnologie, Biochimie Microbienne, Génie des Procédés, Microbiologie Par Sophal TRY Titre : PRODUCTION D’ARÔMES PAR FERMENTATION EN MILIEU SOLIDE Soutenance prévue le 25 Mai 2018 Andrée VOILLEY Pr. émérite, AgroSup Dijon Examinateur Christian LARROCHE Pr, Polytech Clermont-Ferrand, UCA Rapporteur Vincent PHALIP Pr, Université de Lille Rapporteur Yves WACHÉ Pr, AgroSup-Dijon, UBFC Directeur de thèse Joëlle DE-CONINCK Dr, Welience-PPB Dijon, UBFC Codirectrice de thèse Thavarith CHUNHIENG Dr, ITC, Cambodge Codirecteur de thèse Thèse financée par l’Ambassade de France au Cambodge. Production d’arômes par fermentation en milieu solide ©Sophal TRY 2018 RESUME La transposition d’un milieu liquide (FML) à un milieu solide (FMS) de la production de γ- décalactones par fermentation par Yarrowia lipolytica a été étudiée pour la première fois. Dans un premier temps, différentes matrices solides (rafles de maïs, éponges de cellulose, éponges de courgette et graines de ricin) ont été utilisées. L'éponge de courgette était le support sur lequel les lactones étaient produites aux concentrations les plus élevées. La production de lactones a donc été réalisée sur ce support et dans trois types de réacteurs FMS sous différentes conditions d’aérations (sans aération, avec aération statique et avec aération forcée). Une concentration élevée de 5 g/L d’hydroxy-lactone a été détectée dans la condition d’aération statique en fiole à col large. -

Correlating Volatile Compounds, Sensory Attributes, and Quality Parameters in Stored Fresh-Cut Cantaloupe
J. Agric. Food Chem. 2007, 55, 9503–9513 9503 Correlating Volatile Compounds, Sensory Attributes, and Quality Parameters in Stored Fresh-Cut Cantaloupe ,† ‡ JOHN C. BEAULIEU* AND VICKI A. LANCASTER United States Department of Agriculture, Agricultural Research Service, Southern Regional Research Center, Food Processing and Sensory Quality Unit, 1100 Robert E. Lee Boulevard, New Orleans, Louisiana 70124, and Neptune and Company, Inc., 1176 Kimbro Drive, Baton Rouge, Louisiana 70808-6044 Changes in post-cutting volatiles, quality, and sensory attributes during fresh-cut storage (4 °C) of cantaloupe (Cucumis melo L. var. Reticulatus, Naudin, cv. ‘Sol Real’) harvested at four distinct maturities (1/4-, 1/2-, 3/4-, and full-slip) were investigated after 0, 2, 5, 7, 9, 12, and 14 days in a 2-year study. Increased fruity and sweet taste attributes were negatively correlated with percent acetates, aromatic acetates, and total aromatic compounds, and positively correlated with percentage non-acetate esters. Ethyl hexanoate was strongly positively correlated with fruity and sweet taste. Cucurbit, water-like, hardness, cohesiveness, and denseness were positively correlated with percentage acetates, aromatic acetates, and total aromatic compounds, and negatively correlated with percentage non-acetate esters. Several non-acetate esters such as ethyl 2-methyl propanoate, ethyl butanoate, ethyl 2-methyl butanoate, and ethyl hexanoate were negatively (often strongly) correlated with cucurbit. Hardness was positively and strongly correlated with aromatic acetates and all aromatic (benzyl) compounds. In summary, firmer and denser cubes contained more acetates and fewer non-acetate esters. The apparently negative or undesirable attributes cucurbit and water- like were associated with higher acetates and aromatic compounds. -

An Abstract of the Thesis Of
AN ABSTRACT OF THE THESIS OF Anawat Suwanagul for the degree of Doctor of Philosophy in Horticulture presented on March 7. 1996. Title: Ripening Pear Flavor Volatiles: Identification. Biosynthesis and Sensory Perception. Abstract approved: ^ Daryl G. Richardson Volatile compounds of eight pear varieties were isolated and identified by GC/MS. A total of 112 components were identified from headspace of intact fruits, including 47 compounds reported for the first time in pear. The volatile profiles of these pear varieties were characterized by esters, alcohols, hydrocarbons, aldehydes and ketones. Qualitative and quantitative differences of compounds in the profiles are discussed in terms of flavor differences between pear varieties. Analysis of volatiles isolated from eating-ripe pears by direct sniffing GC indicated that a group of nine esters including 2-methylpropyl-, butyl-, pentyl- and hexyl acetates, butyl butanoate, ethyl hexanoate, methyl trans-2, cis-4-, ethyl trans-2, cis-4- and ethyl cis-2, cis-4- decadienoates from 'Bartlett', 'Cornice' and 'Anjou' pear possessed strong, specific pear-like aroma. A second group of esters was also found to possess very positive aroma and can significantly affect overall sensation by serving as 'contributory to flavor compounds'. A newly developed, solid-phase microextraction technique was used to follow changes of volatile production of 'Bartlett', 'Packham's Triumph' and 'Anjou' pears during ripening. The major esters were found to increase at different rates and times during fruit ripening. Highly significant correlations between specific groups of odor- active compounds and pear flavor intensity during ripening indicated the significant contribution of volatiles to overall pear flavor. The effects of harvest maturity on volatiles differed between fruit varieties. -

L 128/5 EN Official Journal of the European Union 16.5.2019
L 128/5 EN Official Journal of the European Union 16.5.2019 Decision of the DECISION OF THE EEA JOINT COMMITTEE No 120/2017 of 7 July 2017 amending Annex I (Veterinary and phytosanitary matters) to the EEA Agreement [2019/727] THE EEA JOINT COMMITTEE, Having regard to the Agreement on the European Economic Area (‘the EEA Agreement’), and in particular Article 98 thereof, Whereas: (1) Commission Implementing Regulation (EU) 2017/53 of 14 December 2016 concerning the authorisation of butan-1-ol, hexan-1-ol, octan-1-ol, nonan-1-ol, dodecan-1-ol, heptan-1-ol, decan-1-ol, pentan-1-ol, ethanol, acetaldehyde, propanal, butanal, pentanal, hexanal, octanal, decanal, dodecanal, nonanal, heptanal, undecanal, 1,1-diethoxyethane, formic acid, acetic acid, propionic acid, valeric acid, hexanoic acid, octanoic acid, decanoic acid, dodecanoic acid, oleic acid, hexadecanoic acid, tetradecanoic acid, heptanoic acid, nonanoic acid, ethyl acetate, propyl acetate, butyl acetate, hexyl acetate, octyl acetate, nonyl acetate, decyl acetate, dodecyl acetate, heptyl acetate, methyl acetate, methyl butyrate, butyl butyrate, pentyl butyrate, hexyl butyrate, octyl butyrate, ethyl decanoate, ethyl hexanoate, propyl hexanoate, pentyl hexanoate, hexyl hexanoate, methyl hexanoate, ethyl formate, ethyl dodecanoate, ethyl tetradecanoate, ethyl nonanoate, ethyl octanoate, ethyl propionate, methyl propionate, ethyl valerate, butyl valerate, ethyl hex-3-enoate, ethyl hexadecanoate, ethyl trans-2-butenoate, ethyl undecanoate, butyl isovalerate, hexyl isobutyrate, methyl 2-methylbutyrate, -

1 Brewing + Distilling Center Beer Ester List
Brewing + Distilling Center Beer Ester List Ester name Formula Odor or occurrence Allyl hexanoate pineapple Benzyl acetate pear, strawberry, jasmine Bornyl acetate pine Butyl acetate apple, honey Butyl butyrate pineapple Butyl propanoate pear drops Ethyl acetate nail polish remover, model paint, model airplane glue Ethyl benzoate sweet, wintergreen, fruity, medicinal, cherry, grape Ethyl butyrate banana, pineapple, strawberry 1 Ethyl hexanoate pineapple, waxy-green banana Ethyl cinnamate cinnamon Ethyl formate lemon, rum, strawberry Ethyl heptanoate apricot, cherry, grape, raspberry Ethyl isovalerate apple Ethyl lactate butter, cream Ethyl nonanoate grape Ethyl pentanoate apple Geranyl acetate geranium Geranyl butyrate cherry Geranyl pentanoate apple Isobutyl acetate cherry, raspberry, strawberry 2 Isobutyl formate raspberry Isoamyl acetate pear, banana (flavoring in Pear drops) Isopropyl acetate fruity Linalyl acetate lavender, sage Linalyl butyrate peach Linalyl formate apple, peach Methyl acetate glue Methyl anthranilate grape, jasmine Methyl benzoate fruity, ylang ylang, feijoa Methyl butyrate (methyl pineapple, apple, strawberry butanoate) 3 Methyl cinnamate strawberry Methyl pentanoate (methyl flowery valerate) Methyl phenylacetate honey Methyl salicylate (oil of Modern root wintergreen) beer, wintergreen, Germolene and Ralgex ointments (UK) Nonyl caprylate orange Octyl acetate fruity-orange Octyl butyrate parsnip Amyl acetate (pentyl apple, banana acetate) Pentyl butyrate (amyl apricot, pear, pineapple butyrate) Pentyl hexanoate (amyl apple, pineapple caproate) Pentyl pentanoate (amyl apple valerate) 4 Propyl acetate pear Propyl hexanoate blackberry, pineapple, cheese, wine Propyl isobutyrate rum Terpenyl butyrate cherry 5 . -

Emission Patterns of Esters and Their Precursors Throughout Ripening
J. AMER.SOC.HORT.SCI. 146(5):297–328. 2021. https://doi.org/10.21273/JASHS05064-21 Emission Patterns of Esters and Their Precursors Throughout Ripening and Senescence in ‘Redchief Delicious’ Apple Fruit and Implications Regarding Biosynthesis and Aroma Perception Alejandra Ferenczi Ministry of Livestock, Agriculture, and Fisheries, Department of Biosecurity, 1476 Montevideo, 11200, Uruguay Nobuko Sugimoto and Randolph M. Beaudry Department of Horticulture, Michigan State University, 1066 Bogue Street, East Lansing, MI 48824 ADDITIONAL INDEX WORDS. AAT, alcohol acyl transferase, sensory, citramalate, fatty acid, Km, Vmax ABSTRACT. The volatile profile of ‘Redchief Delicious’ apple (Malus 3domestica Borkh.) fruit was evaluated at 18 time points from 3 weeks before to 8 weeks after onset of autocatalytic ethylene production to capture the dynamics associated with development from mature green to senescent fruit. Minor amounts of ester production began several days before the onset of ethylene production. Ester production rose rapidly as internal ethylene levels increased beyond 22 nmol·L21 (0.5 mL·L21). Peak ester production roughly coincided with maximum ethylene synthesis, declin- ing thereafter. Ester production was further evaluated according to the acid- (alkanoate) and alcohol- (alkyl) derived portions of the ester. The maximum rate of production for a given ester tended to occur later in development as the chain length of the alcohol-derived portion declined. The production rate for many esters paralleled the rate of ema- nation of their respective alcohol substrates, suggesting that availability of the alcohols limits ester production more than availability of the acid substrates. Combining production rates with sensory descriptors and human sensitivity to individual volatiles permitted approximations of aroma sensations likely engendered by the fruit throughout ripen- ing.