United States Patent Office Patented Aug
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Relative Reactivities of Some Organometallic Compounds Lester D
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1940 Relative reactivities of some organometallic compounds Lester D. Apperson Iowa State College Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Organic Chemistry Commons Recommended Citation Apperson, Lester D., "Relative reactivities of some organometallic compounds " (1940). Retrospective Theses and Dissertations. 13183. https://lib.dr.iastate.edu/rtd/13183 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. NOTE TO USERS This reproduction is the best copy available. UMI BELAflVI REAOflVITIES OP SOME OMANOHSTALlilO COMPOUNDS by Lester D. Apperson A thesis aateltted to the (Graduate Faculty for the Degree of DOCfOR OF PHILOSOPHX Major Subjeot Organic Chemietry Approved! Signature was redacted for privacy. In cha3%e of Major work Signature was redacted for privacy. or Department Signature was redacted for privacy. Dean of Qraduat® College^ Iowa State College 1940 UMI Number: DP12552 INFORMATION TO USERS The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleed-through, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. -

NOTES 14 Decarboxylation Studies. 11. Preparation of Alkyl Phenyl
MARCH, 1963 NOTES 879 concluded that no Grignard reagent is actually formed. g. (0.337 mole) of 2-dimethylaminoethyl chloride, along with the Instead a mechanism for this elimination is suggested separate addition of 125 ml. of tetrahydrofuran. At the end of the exothermic stage the mixture was refluxed 40 min. further. whereby coordination of the metal with nitrogen occurs It was then cooled and unchanged magnesium was removed by resulting in a displacement in the direction shown. decantation of the supernatant liquid without exposure to the atmosphere. The recovered magnesium was washed with ether R R several times, dried, and weighed 4.46 g. (0.184 g.-atom). The 14 n I R-?-CH:!-CHz-Hal R-N-Mg-Hal t. CPH4 Dry Ice trap was found to contain 12.4 g. of liquid which gave, - when distilled through a 6-in. Vigreux column, 11.7 ml. of tetra- hydrofuran, b.p. 64-67', further identified by its infrared spectrum. A 2.7-ml. forerun was not investigated. The bromine from the absorption flasks was combined and distilled through a Further, we note the similarity between the elimina- 6-in. Vigreux column. Isolated was 39.8 g. of ethylene bromide tion described here (I) and that observed in the Cope (0.212 mole), b.p. 127-131", 55.3% based upon the magnesium elimination of N-oxides (11) where retention of con- consumed. It was identified by comparison of its infrared spec- figuration was observed. trum with that of an authentic sample of 1,Zethylene dibromide. The residue of black tar remaining in the distillation flask weighed 10.0 g. -

Reactions of Benzene & Its Derivatives
Organic Lecture Series ReactionsReactions ofof BenzeneBenzene && ItsIts DerivativesDerivatives Chapter 22 1 Organic Lecture Series Reactions of Benzene The most characteristic reaction of aromatic compounds is substitution at a ring carbon: Halogenation: FeCl3 H + Cl2 Cl + HCl Chlorobenzene Nitration: H2 SO4 HNO+ HNO3 2 + H2 O Nitrobenzene 2 Organic Lecture Series Reactions of Benzene Sulfonation: H 2 SO4 HSO+ SO3 3 H Benzenesulfonic acid Alkylation: AlX3 H + RX R + HX An alkylbenzene Acylation: O O AlX H + RCX 3 CR + HX An acylbenzene 3 Organic Lecture Series Carbon-Carbon Bond Formations: R RCl AlCl3 Arenes Alkylbenzenes 4 Organic Lecture Series Electrophilic Aromatic Substitution • Electrophilic aromatic substitution: a reaction in which a hydrogen atom of an aromatic ring is replaced by an electrophile H E + + + E + H • In this section: – several common types of electrophiles – how each is generated – the mechanism by which each replaces hydrogen 5 Organic Lecture Series EAS: General Mechanism • A general mechanism slow, rate + determining H Step 1: H + E+ E El e ctro - Resonance-stabilized phile cation intermediate + H fast Step 2: E + H+ E • Key question: What is the electrophile and how is it generated? 6 Organic Lecture Series + + 7 Organic Lecture Series Chlorination Step 1: formation of a chloronium ion Cl Cl + + - - Cl Cl+ Fe Cl Cl Cl Fe Cl Cl Fe Cl4 Cl Cl Chlorine Ferric chloride A molecular complex An ion pair (a Lewis (a Lewis with a positive charge containing a base) acid) on ch lorine ch loronium ion Step 2: attack of -

Acetophenone Sigma Aldrich.Pdf
SIGMA-ALDRICH sigma-aldrich.com Material Safety Data Sheet Version 4.0 Revision Date 02/27/2010 Print Date 07/08/2011 1. PRODUCT AND COMPANY IDENTIFICATION Product name : Acetophenone Product Number : A10701 Brand : Sigma-Aldrich Company : Sigma-Aldrich 3050 Spruce Street SAINT LOUIS MO 63103 USA Telephone : +1 800-325-5832 Fax : +1 800-325-5052 Emergency Phone # : (314) 776-6555 2. HAZARDS IDENTIFICATION Emergency Overview OSHA Hazards Combustible Liquid, Harmful by ingestion., Irritant GHS Label elements, including precautionary statements Pictogram Signal word Danger Hazard statement(s) H227 Combustible liquid H302 Harmful if swallowed. H318 Causes serious eye damage. Precautionary statement(s) P210 Keep away from heat/sparks/open flames/hot surfaces. - No smoking. P264 Wash skin thoroughly after handling. P270 Do not eat, drink or smoke when using this product. P280 Wear protective gloves/protective clothing/eye protection/face protection. P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor/physician if you feel unwell. P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P310 Immediately call a POISON CENTER or doctor/physician. P330 Rinse mouth. P370 + P378 In case of fire: Use dry sand, dry chemical or alcohol-resistant foam for extinction. P403 + P235 Store in a well-ventilated place. Keep cool. P501 Dispose of contents/container to an approved waste disposal plant. HMIS Classification Health hazard: 2 Flammability: 2 Physical hazards: 0 NFPA Rating Health hazard: 2 Fire: 2 Reactivity Hazard: 0 Potential Health Effects Sigma-Aldrich - A10701 Page 1 of 6 Inhalation May be harmful if inhaled. -

ACETOPHENONE Method No. PV2003 Target Concentration
ACETOPHENONE Method no. PV2003 Target concentration: 100 ppm (491 mg/m3) Procedure: Samples are collected by drawing a known volume of air through a Tenax GC tube. Samples are desorbed with a solvent mixture of (5:95) Isopropanol:Carbon Disulfide (IPA):(CS2) and analyzed by gas chromatography with a flame ionization detector. Recommended air volume 120 minutes at 0.1 L/min (12 L) and sampling rate: Status of Method: Partially Validated. This method has been only partially evaluated and is presented for information and trial use. July 1982 Wayne Potter Service Branch 1 OSHA Salt Lake Technical Center Salt Lake City UT-84115 1 of 8 1 General Discussion 1.1 Background 1.1.1 History of procedure Recently, the OSHA Analytical Laboratory received a set of field samples that required analysis for acetophenone. The air samples had been collected on charcoal tubes and isopropanol impingers. Desorption studies were done on charcoal tubes using acetone, carbon disulfide, 5:95 isopropanol:carbon disulfide, and methylene chloride as desorbing solvents. The best results were obtained with 5:95 isopropanol:carbon disulfide, but the recovery was only 68%, which indicated that charcoal tubes should not be recommended for the collection of acetophenone air samples. An evaluation of isopropanol impingers was not performed because it was preferable to find an adsorbent procedure for sample collection. NIOSH method 291 for α -chloroacetophenone uses Tenax GC tubes. The Tenax GC tubes were tried for acetophenone, and appeared to be a suitable method of collection. 1.1.2 Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy.) Acetophenone is a narcotic in high concentrations (Ref. -
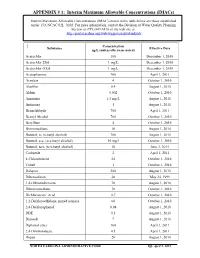
APPENDIX # 1: Interim Maximum Allowable Concentrations (Imacs)
APPENDIX # 1: Interim Maximum Allowable Concentrations (IMACs) Interim Maximum Allowable Concentrations (IMAC) shown in the table below are those established under 15A NCAC 02L .0202. For more information, contact the Division of Water Quality Planning Section at (919) 807-6416 or the web site at: http://portal.ncdenr.org/web/wq/ps/csu/gwstandards Concentration Substance Effective Date ug/L (unless otherwise noted) Acetochlor 100 December 1, 2010 Acetochlor ESA 1 mg/L December 1, 2010 Acetochlor OXA 1 mg/L December 1, 2010 Acetophenone 700 April 1, 2011 Acrolein 4 October 1, 2010 Alachlor 0.4 August 1, 2010 Aldrin 0.002 October 1, 2010 Ammonia 1.5 mg/L August 1, 2010 Antimony 1 August 1, 2010 Benzaldehyde 700 April 1, 2011 Benzyl Alcohol 700 October 1, 2010 Beryllium 4 October 1, 2010 Bromomethane 10 August 1, 2010 Butanol, n- (n-butyl alcohol) 700 August 1, 2010 Butanol, sec- (sec-butyl alcohol) 10 mg/l October 1, 2010 Butanol, tert- (tert-butyl alcohol) 10 June 1, 2011 Carbazole 2 April 1, 2011 4-Chlorotoluene 24 October 1, 2010 Cobalt 1 October 1, 2010 Dalapon 200 August 1, 2010 Dibenzofuran 28 May 24, 1999 1,4-Dibromobenzene 70 August 1, 2010 Dibromomethane 70 October 1, 2010 Dichloroacetic Acid 0.7 October 1, 2010 1,2-Dichloroethylene, mixed isomers 60 October 1, 2010 2,4-Dichlorophenol 0.98 August 1, 2010 DDE 0.1 August 1, 2010 Dinoseb 7 August 1, 2010 Diphenyl ether 100 April 1, 2011 2,4-Dinitrotoluene 0.1 April 1, 2011 Diquat 20 August 1, 2010 NORTH CAROLINA ADMINISTRATIVE CODE Eff. -

DESIGN, SYNTHESIS, CHARACTERIZATION of SOME NEW SUBSTITUTED CHALCONES and STUDIES THEIR ANTIMICROBIAL ACTIVITIES Zakaria H
[Aiube* et al., 5(6): June, 2016] ISSN: 2277-9655 IC™ Value: 3.00 Impact Factor: 4.116 IJESRT INTERNATIONAL JOURNAL OF ENGINEERING SCIENCES & RESEARCH TECHNOLOGY DESIGN, SYNTHESIS, CHARACTERIZATION OF SOME NEW SUBSTITUTED CHALCONES AND STUDIES THEIR ANTIMICROBIAL ACTIVITIES Zakaria H. Aiube*, Ali H. Samir, Israa SH. A- R. Al- Kadi * Department of Chemistry, college of Education for pure Science-Ibn, Al- Haitham, University, Baghdad, Iraq DOI: 10.5281/zenodo.55982 ABSTRACT Eight designed chalcones, named [1(p-benzenesulphonamidophenyl)-3-p-chloro-2-propene-1-one][2], [1(p- benzenesulphonamidophenyl)-3-p-nitro-2-propene-1-one][3], [1(4-Ureido)phenyl-3-p-chlorophenyl-2-propene- 1-one][5], [1(4-Ureido)phenyl-3-p-nitroophenyl-2-propene-1-one][6], [1(4(p-N-methylaminophenyl)azophenyl- 3-p-chlorophenyl-2-propene-1-one][8], [1(4(p-N-methylaminophenyl)azophenyl-3-p-nitroophenyl-2-propene-1- one][9], [1(p-aminophenyl)-3-p-chlorophenyl-2-propene-1-one][10] and [1(p-aminophenyl)-3-p-nitrophenyl-2- propene-1-one][11], were synthesised by condensation of synthesised p-acetylphenylbenzene- sulphonamide, p- acetylphenylurea and p-acetyl-p'-(N-methylamino)azobenzene, with p-chlorobenzaldehyde and p nitrobenzaldehyde in basic media respectively. All synthesised compounds are characterized by its melting points, FTIR, 1H NMR, 13C NMR and Mass spectral analysis. All synthesised compounds are examined their antimicrobial activities against Gram-Ve bactria (Serratia marcescens, Pseudmonas aeroginosa) and Gram+Ve bacterial (Stphylococcus aureus, Streptococcus pyogenes), and Candida albicans fungi. Result showed good to moderate inhibition effect against some bacteria and fungi, in comparison with some pharmaceutical antibiotic and antifungal treatments like Cephalexin, Amoxicillin, Tetracycline, Lincomycine, Nystatine and Fluconazole respectively. -

List of Lists
United States Office of Solid Waste EPA 550-B-10-001 Environmental Protection and Emergency Response May 2010 Agency www.epa.gov/emergencies LIST OF LISTS Consolidated List of Chemicals Subject to the Emergency Planning and Community Right- To-Know Act (EPCRA), Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) and Section 112(r) of the Clean Air Act • EPCRA Section 302 Extremely Hazardous Substances • CERCLA Hazardous Substances • EPCRA Section 313 Toxic Chemicals • CAA 112(r) Regulated Chemicals For Accidental Release Prevention Office of Emergency Management This page intentionally left blank. TABLE OF CONTENTS Page Introduction................................................................................................................................................ i List of Lists – Conslidated List of Chemicals (by CAS #) Subject to the Emergency Planning and Community Right-to-Know Act (EPCRA), Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) and Section 112(r) of the Clean Air Act ................................................. 1 Appendix A: Alphabetical Listing of Consolidated List ..................................................................... A-1 Appendix B: Radionuclides Listed Under CERCLA .......................................................................... B-1 Appendix C: RCRA Waste Streams and Unlisted Hazardous Wastes................................................ C-1 This page intentionally left blank. LIST OF LISTS Consolidated List of Chemicals -

LIST of ANNEXURES SR. NO. NAME of ANNEXURE I List of Products with Their Production Capacity II Layout Map of the Plant III Brie
LIST OF ANNEXURES SR. NO. NAME OF ANNEXURE I List of products with their production capacity II Layout Map of the Plant III Brief Manufacturing Process Descrip tion with Chemical and Mass Balance IV Details of Water Consumption Wastewater Generation V Effluent Treatment Scheme VI Details of Hazardous /Solid Waste Generation, Handling and Disposal VII Details of Air pollution Control System (Stack & Vent) VI II Details of Hazardous Chemicals Storage & Handling IX Socio-economic Impacts X Proposed Terms of Reference for EIA studies ANNEXURE – 1 LIST OF PRODUCTS WITH THEIR PRODUCTION CAPACITY Production (MT/Month) LD Sr. Product Name CAS No. Proposed Proposed 50(mg/Kg) No. Existing Total Nytrosyl Sulphuric Acid (from 7782-78-7 467.92 2140 1 132.08 600 Sulfur) * Nytrosyl Sulphuric Acid (from 7782-78-7 00 2140 2 Sodium Thio Sulphate Solution and 468 468 Spent Acid (H2SO4)) ** 3.1 4-Met hyl Acetophenone 122 -00 -9 1400 00 60 3.2 Acetyl Furan 1192-62-7 60 1130 4.0 Diethyl Ketone 96 -22 -0 00 179 179 2737 5.1 Methyl Propyl Ketone 107 -87 -9 20001 5.2 Dipropyl Ketone 123-19-3 00 100 100 20001 5.3 Propiophenone 93-55-0 4500 6 Alpha Nitro Napthalene 86-57-7 00 100 100 2560 7 Alpha Napthylamine 134 -32 -7 00 165 165 2000 Phenyl Alpha Naphthylamine 90-30-2 90 90 2000 8 00 (PANA) Epichlorohydrin Based Polyamide 106-89-8 116 116 1580 9 00 resin 10 2,4-Dichloro Acetophenone 2234-16-4 00 60 60 1800 98-86-2 60 60 815 11 Acetophenone 00 IGBA - 3-(2- 75143-89-4 68.2652 68.2652 3580 12.1 methylpropyl)pentanedioic acid 00 KSM - 3-(2-amino-2-oxoethyl)-5- 181289-15- -

Three-Dimensional Docking of Alcohols to Ketones: an Experimental Benchmark Based on Cite This: Phys
PCCP View Article Online PAPER View Journal | View Issue Three-dimensional docking of alcohols to ketones: an experimental benchmark based on Cite this: Phys. Chem. Chem. Phys., 2020, 22,2870 acetophenone solvation energy balances† C. Zimmermann, H. C. Gottschalk and M. A. Suhm * The two hydrogen bond solvation sites exhibited by the carbonyl group in acetophenone are influenced by alkylation of the methyl group in both the acetophenone and in the prototype solvent methanol, largely due to London dispersion forces. Phenyl docking and alkyl docking preferences can be realized at will by appropriate substitution. In particular, cyclopropylation helps to stabilize the opposite phenyl docking site. In all cases, the energy gap is small enough to allow for a simultaneous detection even under low temperature conditions. This density functional prediction is checked experimentally by jet FTIR spectroscopy and largely confirmed. A spurious out-of-plane solvation preference predicted for cyclopropylphenylketone with tert- Received 12th November 2019, butyl alcohol by B3LYP-D3 calculations is not confirmed experimentally. It is unlikely that this discrepancy is Creative Commons Attribution 3.0 Unported Licence. Accepted 2nd January 2020 due to zero-point energy effects. Instead, the second most stable alkyl-side solvation motif predicted with a DOI: 10.1039/c9cp06128b more in-plane coordination is found in the jet expansion. Overall, the ability of carbonyl solvation balances to benchmark subtle electronic structure effects for non-covalent interactions -

Acetophenone
Acetophenone 98-86-2 Hazard Summary Acetophenone is used for fragrance in soaps and perfumes, as a flavoring agent in foods, and as a solvent for plastics and resins. Acute (short-term) exposure to acetophenone vapor may produce skin irritation and transient corneal injury in humans. No information is available on the chronic (long-term), reproductive, developmental, or carcinogenic effects of acetophenone in humans. EPA has classified acetophenone as a Group D, not classifiable as to human carcinogenicity. Please Note: The main sources of information for this fact sheet are EPA's Integrated Risk Information System (IRIS) (5), which contains information on oral chronic toxicity of acetophenone and the RfD, and EPA's Health and Environmental Effects Document for Acetophenone (2). Other secondary sources include the Hazardous Substances Data Bank (HSDB) (3), a database of summaries of peer-reviewed literature, and the Registry of Toxic Effects of Chemical Substances (RTECS) (4), a database of toxic effects that are not peer reviewed. Uses Acetophenone is used in perfumery as a fragrance ingredient in soaps, detergents, creams, lotions, and perfumes; as a flavoring agent in foods, nonalcoholic beverages, and tobacco; as a specialty solvent for plastics and resins; as a catalyst for the polymerization of olefins; and in organic syntheses as a photosensitizer. (2,3,6) Sources and Potential Exposure Occupational exposure to acetophenone may occur during its manufacture and use. (1) Acetophenone has been detected in ambient air and drinking water; exposure of the general public may occur through the inhalation of contaminated air or the consumption of contaminated water. (2) Assessing Personal Exposure Hippuric acid may be monitored in the urine to determine whether or not exposure to acetophenone has occurred. -

Project Feasibility Report
PRE FEASIBILITY REPORT For PROPOSED BULK DRUGS AND BULK DRUG INTERMEDIATES IN EXISTING THINERS FORMULATION UNIT of M/s. ANJU LIFE SCIENCE Plot No. 6007/1, GIDC, Ankleshwar – 393 002, Dist. Bharuch, Gujarat 0 CONTENTS Sr. No. Description Page No. 1. Executive Summary 2 2. Introduction of the Project/Background information 21 3. Project Description 22 4. Site Analysis 28 5. Planning Brief 29 6. Proposed Infrastructure 30 7. Rehabilitation and resettlement (R & R) Plan 31 8. Project Schedule & Cost Estimates 31 9. Analysis of Proposal (Final Recommendations) 32 1 1.0 EXECUTIVE SUMMARY 1.1 Company Profile M/s. Anju Life Science engages in manufacturing of formulation of thinners unit at Plot No. 6007/1, GIDC, Ankleshwar - 393 002, Dist: Bharuch, Gujarat, INDIA. Now, company is going for new bulk drugs, bulk drug intermediates & solvent distillation (in-house common group facility) within existing premises. 1.2 Project Details 1.2.1 Products along with Production Capacity Sr. Products Capacity CAS No. No. (MT/Month) Existing Additional Total Existing 1. Formulation of Thinners/Reducers 500 -- 500 Proposed 1 Furosemide -- 5 5 54-31-9 2 Diaminomethyleneamino (1-amino-1- -- 100 100 -- iminomethylene) thiomethyl thiozole dihydrochloride [ITU] 3 N-Sulfomyl-3-chloropropionamide -- 100 100 123-92-2 hydrochloride[IF] 4 Famotidine -- 5 5 105-68-0 5 Fomepizole -- 14205-39-1 6 Colsevelam hydrochloride -- 88150-62-3 7 Glimepiride -- 93479-97-1 8 Betahistine Dihydrochloride -- 106649-95-0 9 Adapalene -- 76824-35-6 10 Telmisartan -- 152751-57-0 11 Tapentadol