A Study of Chloretone and Related Compounds Earle Alexander Tompkins University of Massachusetts Amherst
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Potassium Hydroxide Cas N°: 1310-58-3
OECD SIDS POTASSIUM HYDROXIDE FOREWORD INTRODUCTION POTASSIUM HYDROXIDE CAS N°: 1310-58-3 UNEP PUBLICATIONS 1 OECD SIDS POTASSIUM HYDROXIDE SIDS Initial Assessment Report For SIAM 13 Bern, Switzerland, 6-9 November 2001 1. Chemical Name: Potassium hydroxide 2. CAS Number: 1310-58-3 3. Sponsor Country: Belgium Dr. Thaly LAKHANISKY J. Wytsman 16 B-1050 Brussels, Belgium Tel : + 32 2 642 5104 Fax : +32 2 642 5224 E-mail : [email protected] 4. Shared Partnership with: ICCA (Tessenderlo Chemie NV) 5. Roles/Responsibilities of the Partners: · Name of industry sponsor /consortium · Process used 6. Sponsorship History · How was the chemical or In 2001, ICCA (Tessenderlo Chemie NV)) had proposed sponsor category brought into the and prepared draft documents(Dossier, SIAR, SIAP). It was OECD HPV Chemicals submitted to the SIDS contact point of Belgium on May 2001. Programme? The draft documents were revised by Belgium after discussion with Tessenderlo Chemie NV. The revised draft was discussed in detail with Tessenderlo Chemie NV on June and July 2001. After agreement, the documents were finalized and the checklist was developed by jointly by Belgium and Tessenderlo Chemie NV 7. Review Process Prior to the SIAM: 8. Quality check process: 9. Date of Submission: 10. Date of last Update: February 2002 11. Comments: No testing 2 UNEP PUBLICATIONS OECD SIDS POTASSIUM HYDROXIDE SIDS INITIAL ASSESSMENT PROFILE CAS No. 1310-58-3 Chemical Name Potassium hydroxide Structural Formula KOH RECOMMENDATIONS The chemical is currently of low priority for further work. SUMMARY CONCLUSIONS OF THE SIAR Human Health Solid KOH is corrosive. -

Exposure to Potassium Hydroxide Can Cause Headache, Eye Contact Dizziness, Nausea and Vomiting
Right to Know Hazardous Substance Fact Sheet Common Name: POTASSIUM HYDROXIDE Synonyms: Caustic Potash; Lye; Potassium Hydrate CAS Number: 1310-58-3 Chemical Name: Potassium Hydroxide (KOH) RTK Substance Number: 1571 Date: May 2001 Revision: January 2010 DOT Number: UN 1813 Description and Use EMERGENCY RESPONDERS >>>> SEE LAST PAGE Potassium Hydroxide is an odorless, white or slightly yellow, Hazard Summary flakey or lumpy solid which is often in a water solution. It is Hazard Rating NJDOH NFPA used in making soap, as an electrolyte in alkaline batteries and HEALTH - 3 in electroplating, lithography, and paint and varnish removers. FLAMMABILITY - 0 Liquid drain cleaners contain 25 to 36% of Potassium REACTIVITY - 1 Hydroxide. CORROSIVE POISONOUS GASES ARE PRODUCED IN FIRE DOES NOT BURN Reasons for Citation Hazard Rating Key: 0=minimal; 1=slight; 2=moderate; 3=serious; f Potassium Hydroxide is on the Right to Know Hazardous 4=severe Substance List because it is cited by ACGIH, DOT, NIOSH, NFPA and EPA. f Potassium Hydroxide can affect you when inhaled and by f This chemical is on the Special Health Hazard Substance passing through the skin. List. f Potassium Hydroxide is a HIGHLY CORROSIVE CHEMICAL and contact can severely irritate and burn the skin and eyes leading to eye damage. f Contact can irritate the nose and throat. f Inhaling Potassium Hydroxide can irritate the lungs. SEE GLOSSARY ON PAGE 5. Higher exposures may cause a build-up of fluid in the lungs (pulmonary edema), a medical emergency. FIRST AID f Exposure to Potassium Hydroxide can cause headache, Eye Contact dizziness, nausea and vomiting. -

PCTM 17 ISSUED-1996 Method to Determine Rosin Acids in Tall Oil
PCTM 17 ISSUED-1996 Method to determine rosin acids in tall oil Scope 3. Methyl sulfuric acid solution, 20% - Caution: This method covers the determination of rosin acids in Slowly pour 100 g of concentrated sulfuric acid (- tall oils containing more than 15% rosin acids. 96%), while stirring constantly, into 400 g of methanol. This method may not be applicable to adducts or 4 . Thymol blue indicator - Weigh 0.1 g thymol blue in derivatives of tall oils, or other naval stores products. 100 mL methanol. Fatty acids are esterified by methanol in the presence of sulfuric Sample Preparation acid catalyst, and rosin acids are determined by titration after neutralization of the sulfuric acid. 1. Dissolve 5 ± 0.5 g of sample, weighed to the nearest 0.001 g, into a 250-m1. Erlenmeyer flask. Apparatus 2. Add 100 mL of methanol and swirl to dissolve. 3. Add 5.0 mL of methyl sulfuric acid solution. 1. Beaker, tall-form, 300-mL capacity. 4. Connect the flask to the condenser and reflux for 2. Buret, 50-mL capacity with 0.1-mL divisions. 30 minutes. Allow the flask to cool to Electronic burets are preferable for increased approximately room temperature. accuracy and precision. 3. Erlenmeyer flask, 250-mL flat-bottom fitted with a Method A - Potentiometric Titration condenser. 4. pH meter, capable of reading ± 0.1 pH over a 1. Titrate with the KOH solution to a fixed pH of range of pH 1 to pH 13 in alcoholic "solutions. 4.0, the first end point. 5. Pipet, 5-mL. -

SAFETY DATA SHEET Potassium Hydroxide, Liquid 45-50% Revision Date: 2020-06-15 Version: 1.0
SAFETY DATA SHEET Potassium Hydroxide, Liquid 45-50% Revision date: 2020-06-15 Version: 1.0 1. PRODUCT AND COMPANY IDENTIFICATION Product Identifier Product Name: Potassium Hydroxide, Liquid 45-50% Synonyms: Chemical manufacturing, fertilizer, batteries, soaps Product Form: Liquid Recommended use of the chemical and restrictions on use Recommended Use: Professional use, Industrial use. Chemical manufacturing, fertilizer, batteries, soaps Restrictions on Use: Use as recommended by the label Details of the supplier and of the safety data sheet Supplier Tersus Environmental, LLC 1116 Colonial Club Rd Wake Forest, NC 27587 Phone: +1-919-453-5577 Email: [email protected] Contact Person David F. Alden Phone: +1-919-453-5577 x2002 Email: [email protected] Emergency telephone number For leak, fire, spill or accident emergencies, call: +1-919-453-5577 (Tersus Office Hours, 8:00 AM to 5:00 PM Eastern) +1-800-424-9300 (Chemtrec 24 Hour Service – Emergency Only) +1-703-527-3887 (Chemtrec Outside United States 24 Hour Service – Emergency Only) +1-919-638-7892 Gary M. Birk (Outside office hours) 2. HAZARD IDENTIFICATION Relevant identified uses of the substance or mixture GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) GHS label elements, including precautionary statements: Signal Word: Danger Pictogram(s): GHS05 GHS07 Page 1 of 10 Potassium Hydroxide, Liquid 45-50% Revision date: 2020-06-15 Version: 1.0 Hazard statement H290 May be corrosive to metals. H302 Harmful if swallowed. H314 Causes severe skin burns and eye damage H318 Causes serious eye damage. H402 Harmful to aquatic life. Precautionary statement P234 Keep only in original container. -

Reactions of Benzene & Its Derivatives
Organic Lecture Series ReactionsReactions ofof BenzeneBenzene && ItsIts DerivativesDerivatives Chapter 22 1 Organic Lecture Series Reactions of Benzene The most characteristic reaction of aromatic compounds is substitution at a ring carbon: Halogenation: FeCl3 H + Cl2 Cl + HCl Chlorobenzene Nitration: H2 SO4 HNO+ HNO3 2 + H2 O Nitrobenzene 2 Organic Lecture Series Reactions of Benzene Sulfonation: H 2 SO4 HSO+ SO3 3 H Benzenesulfonic acid Alkylation: AlX3 H + RX R + HX An alkylbenzene Acylation: O O AlX H + RCX 3 CR + HX An acylbenzene 3 Organic Lecture Series Carbon-Carbon Bond Formations: R RCl AlCl3 Arenes Alkylbenzenes 4 Organic Lecture Series Electrophilic Aromatic Substitution • Electrophilic aromatic substitution: a reaction in which a hydrogen atom of an aromatic ring is replaced by an electrophile H E + + + E + H • In this section: – several common types of electrophiles – how each is generated – the mechanism by which each replaces hydrogen 5 Organic Lecture Series EAS: General Mechanism • A general mechanism slow, rate + determining H Step 1: H + E+ E El e ctro - Resonance-stabilized phile cation intermediate + H fast Step 2: E + H+ E • Key question: What is the electrophile and how is it generated? 6 Organic Lecture Series + + 7 Organic Lecture Series Chlorination Step 1: formation of a chloronium ion Cl Cl + + - - Cl Cl+ Fe Cl Cl Cl Fe Cl Cl Fe Cl4 Cl Cl Chlorine Ferric chloride A molecular complex An ion pair (a Lewis (a Lewis with a positive charge containing a base) acid) on ch lorine ch loronium ion Step 2: attack of -

Potassium Hydroxide Safety Data Sheet According to Federal Register / Vol
Potassium Hydroxide Safety Data Sheet according to Federal Register / Vol. 77, No. 58 / Monday, March 26, 2012 / Rules and Regulations Date of issue: 10/09/2004 Revision date: 02/06/2018 Supersedes: 02/06/2018 Version: 1.1 SECTION 1: Identification 1.1. Identification Product form : Substance Substance name : Potassium Hydroxide CAS-No. : 1310-58-3 Product code : LC19190 Formula : KOH Synonyms : caustic potash / caustic potash dry / caustic potash, dry solid, flake, bead or granular / caustic potash, solid / caustic potash,solid / hydrate of potash / hydrate of potassium / hydroxide of potash / hydroxide of potassium / lye (=potassium hydroxide) / potash / potash hydrate / potash lye / potassium hydrate / potassium hydroxide (K(OH)) / potassium hydroxide dry / potassium hydroxide pellets / potassium hydroxide, dry solid, flake, bead or granular / potassium hydroxide, electrolytical, solid / potassium hydroxide, solid / Potassium hydroxide, solid / potassium lye 1.2. Recommended use and restrictions on use Use of the substance/mixture : For laboratory and manufacturing use only. Recommended use : Laboratory chemicals Restrictions on use : Not for food, drug or household use 1.3. Supplier LabChem Inc Jackson's Pointe Commerce Park Building 1000, 1010 Jackson's Pointe Court Zelienople, PA 16063 - USA T 412-826-5230 - F 724-473-0647 [email protected] - www.labchem.com 1.4. Emergency telephone number Emergency number : CHEMTREC: 1-800-424-9300 or 011-703-527-3887 SECTION 2: Hazard(s) identification 2.1. Classification of the substance or mixture GHS-US classification Acute toxicity (oral) H302 Harmful if swallowed Category 4 Skin corrosion/irritation H314 Causes severe skin burns and eye damage Category 1A Hazardous to the aquatic H402 Harmful to aquatic life environment - Acute Hazard Category 3 Full text of H statements : see section 16 2.2. -

Acetophenone Sigma Aldrich.Pdf
SIGMA-ALDRICH sigma-aldrich.com Material Safety Data Sheet Version 4.0 Revision Date 02/27/2010 Print Date 07/08/2011 1. PRODUCT AND COMPANY IDENTIFICATION Product name : Acetophenone Product Number : A10701 Brand : Sigma-Aldrich Company : Sigma-Aldrich 3050 Spruce Street SAINT LOUIS MO 63103 USA Telephone : +1 800-325-5832 Fax : +1 800-325-5052 Emergency Phone # : (314) 776-6555 2. HAZARDS IDENTIFICATION Emergency Overview OSHA Hazards Combustible Liquid, Harmful by ingestion., Irritant GHS Label elements, including precautionary statements Pictogram Signal word Danger Hazard statement(s) H227 Combustible liquid H302 Harmful if swallowed. H318 Causes serious eye damage. Precautionary statement(s) P210 Keep away from heat/sparks/open flames/hot surfaces. - No smoking. P264 Wash skin thoroughly after handling. P270 Do not eat, drink or smoke when using this product. P280 Wear protective gloves/protective clothing/eye protection/face protection. P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor/physician if you feel unwell. P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P310 Immediately call a POISON CENTER or doctor/physician. P330 Rinse mouth. P370 + P378 In case of fire: Use dry sand, dry chemical or alcohol-resistant foam for extinction. P403 + P235 Store in a well-ventilated place. Keep cool. P501 Dispose of contents/container to an approved waste disposal plant. HMIS Classification Health hazard: 2 Flammability: 2 Physical hazards: 0 NFPA Rating Health hazard: 2 Fire: 2 Reactivity Hazard: 0 Potential Health Effects Sigma-Aldrich - A10701 Page 1 of 6 Inhalation May be harmful if inhaled. -

Potassium Hydroxide, 0.1N (0.1M) in Ethanol Safety Data Sheet According to Federal Register / Vol
Potassium Hydroxide, 0.1N (0.1M) in Ethanol Safety Data Sheet according to Federal Register / Vol. 77, No. 58 / Monday, March 26, 2012 / Rules and Regulations Date of issue: 12/19/2013 Revision date: 02/06/2018 Supersedes: 02/06/2018 Version: 1.3 SECTION 1: Identification 1.1. Identification Product form : Mixtures Product name : Potassium Hydroxide, 0.1N (0.1M) in Ethanol Product code : LC19310 1.2. Recommended use and restrictions on use Use of the substance/mixture : For laboratory and manufacturing use only. Recommended use : Laboratory chemicals Restrictions on use : Not for food, drug or household use 1.3. Supplier LabChem Inc Jackson's Pointe Commerce Park Building 1000, 1010 Jackson's Pointe Court Zelienople, PA 16063 - USA T 412-826-5230 - F 724-473-0647 [email protected] - www.labchem.com 1.4. Emergency telephone number Emergency number : CHEMTREC: 1-800-424-9300 or 011-703-527-3887 SECTION 2: Hazard(s) identification 2.1. Classification of the substance or mixture GHS-US classification Flammable liquids H225 Highly flammable liquid and vapour Category 2 Serious eye damage/eye H319 Causes serious eye irritation irritation Category 2A Carcinogenicity Category H350 May cause cancer 1A Reproductive toxicity H361 Developmental toxicity (oral) Category 2 Specific target organ H370 Causes damage to organs (central nervous system, optic nerve) (oral, Dermal) toxicity (single exposure) Category 1 Full text of H statements : see section 16 2.2. GHS Label elements, including precautionary statements GHS-US labeling Hazard pictograms (GHS-US) : GHS02 GHS07 GHS08 Signal word (GHS-US) : Danger Hazard statements (GHS-US) : H225 - Highly flammable liquid and vapour H319 - Causes serious eye irritation H350 - May cause cancer H361 - Developmental toxicity (oral) H370 - Causes damage to organs (central nervous system, optic nerve) (oral, Dermal) Precautionary statements (GHS-US) : P201 - Obtain special instructions before use. -

ACETOPHENONE Method No. PV2003 Target Concentration
ACETOPHENONE Method no. PV2003 Target concentration: 100 ppm (491 mg/m3) Procedure: Samples are collected by drawing a known volume of air through a Tenax GC tube. Samples are desorbed with a solvent mixture of (5:95) Isopropanol:Carbon Disulfide (IPA):(CS2) and analyzed by gas chromatography with a flame ionization detector. Recommended air volume 120 minutes at 0.1 L/min (12 L) and sampling rate: Status of Method: Partially Validated. This method has been only partially evaluated and is presented for information and trial use. July 1982 Wayne Potter Service Branch 1 OSHA Salt Lake Technical Center Salt Lake City UT-84115 1 of 8 1 General Discussion 1.1 Background 1.1.1 History of procedure Recently, the OSHA Analytical Laboratory received a set of field samples that required analysis for acetophenone. The air samples had been collected on charcoal tubes and isopropanol impingers. Desorption studies were done on charcoal tubes using acetone, carbon disulfide, 5:95 isopropanol:carbon disulfide, and methylene chloride as desorbing solvents. The best results were obtained with 5:95 isopropanol:carbon disulfide, but the recovery was only 68%, which indicated that charcoal tubes should not be recommended for the collection of acetophenone air samples. An evaluation of isopropanol impingers was not performed because it was preferable to find an adsorbent procedure for sample collection. NIOSH method 291 for α -chloroacetophenone uses Tenax GC tubes. The Tenax GC tubes were tried for acetophenone, and appeared to be a suitable method of collection. 1.1.2 Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy.) Acetophenone is a narcotic in high concentrations (Ref. -

Potassium Hydroxide (P6310)
Potassium hydroxide ACS Reagent Product Number P 6310 Store at Room Temperature 22,147-3 is an exact replacement for P 6310 Product Description Preparation Instructions Molecular Formula: KOH This product is soluble in water (100 mg/ml), yielding a Molecular Weight: 56.11 clear, colorless solution. Potassium hydroxide is also CAS Number: 1310-58-3 soluble in alcohol (1 part in 3) and glycerol (1 part Melting point: 360 °C, 380 °C (anhydrous)1 in 2.5). The dissolution of potassium hydroxide in water or alcohol is a highly exothermic (heat- This product is in the form of pellets. It is designated producing) process.1 as ACS Reagent grade, and meets the specifications of the American Chemical Society (ACS) for reagent Storage/Stability chemicals. Potassium hydroxide rapidly absorbs carbon dioxide and water from the air and deliquesces.1 Potassium Potassium hydroxide (KOH) is a caustic reagent that is hydroxide solutions should be stored in plastic bottles widely used to neutralize acids and prepare potassium (polyethylene or polypropylene). KOH solutions will salts of reagents. It is used in a variety of large-scale etch glass over a period of just a few days. applications, such as the manufacture of soap, the mercerizing of cotton, electroplating, photoengraving, References and lithography.1 1. The Merck Index, 12th ed., Entry# 7806. 2. Philip, N. S., and Green, D. M., Recovery and Potassium hydroxide is used in the analysis of bone enhancement of faded cleared and double stained and cartilage samples by histology.2,3 A protocol for specimens. Biotech. Histochem., 75(4), 193-196 the amplification of DNA from single cells by PCR that (2000). -

CHANGES in Ph ASSOCIATED with the APPLICATION of AMMONIA and POTASSIUM HYDROXIDE to DIAPAUSING EGGS of TELEOGRYLLUS OOMMODUS (WALK.) (ORTHOPTERA: GRYLLIDAE)
CHANGES IN pH ASSOCIATED WITH THE APPLICATION OF AMMONIA AND POTASSIUM HYDROXIDE TO DIAPAUSING EGGS OF TELEOGRYLLUS OOMMODUS (WALK.) (ORTHOPTERA: GRYLLIDAE) By T. W. HOGAN* [Manuscript received September 9, 1964] Summary Diapausing eggs of T. commodus were exposed to a:mmonia evolved from solutions of ammonium hydroxide in desiccators for specific periods. It was found that the pH of the egg contents increased with increase in concentration of the ammonium hydroxide and the period of exposure. Toxic symptoms and mortality of eggs were associated with a rise of pH exceeding 0·4 of a unit. This occurred at the higher concentrations, viz. exposure for 3 days to O· 3M or for 2-3 days to 0 ·IM ammonium hydroxide. The most favourable effect on the termination of diapause was with exposure for 3 days to O· 01M ammonium hydroxide, which raised the pH to 7· O. This was also the highest non-toxic concentration. On the basis that the rise in pH might be the causal factor for the termination of diapause, the effect of potassium hydroxide solutions on the pH of the eggs was tested and found to be moderately effective both in this regard and in accelerating the rate of termination of diapause. However, there was some termination of diapause before any significant rise in pH occurred. It appears, therefore, that the action of ammonia and of potassium hydroxide must have a common factor other than raising the pH of the egg contents. 1. INTRODUCTION It has been shown that when diapausing eggs of Teleogryllu8 commodu8 (Walk.) are exposed to ammonia evolved from solutions of ammonium hydroxide for an adequate period, diapause is terminated in a high proportion of the eggs. -
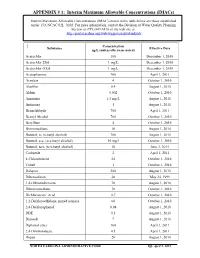
APPENDIX # 1: Interim Maximum Allowable Concentrations (Imacs)
APPENDIX # 1: Interim Maximum Allowable Concentrations (IMACs) Interim Maximum Allowable Concentrations (IMAC) shown in the table below are those established under 15A NCAC 02L .0202. For more information, contact the Division of Water Quality Planning Section at (919) 807-6416 or the web site at: http://portal.ncdenr.org/web/wq/ps/csu/gwstandards Concentration Substance Effective Date ug/L (unless otherwise noted) Acetochlor 100 December 1, 2010 Acetochlor ESA 1 mg/L December 1, 2010 Acetochlor OXA 1 mg/L December 1, 2010 Acetophenone 700 April 1, 2011 Acrolein 4 October 1, 2010 Alachlor 0.4 August 1, 2010 Aldrin 0.002 October 1, 2010 Ammonia 1.5 mg/L August 1, 2010 Antimony 1 August 1, 2010 Benzaldehyde 700 April 1, 2011 Benzyl Alcohol 700 October 1, 2010 Beryllium 4 October 1, 2010 Bromomethane 10 August 1, 2010 Butanol, n- (n-butyl alcohol) 700 August 1, 2010 Butanol, sec- (sec-butyl alcohol) 10 mg/l October 1, 2010 Butanol, tert- (tert-butyl alcohol) 10 June 1, 2011 Carbazole 2 April 1, 2011 4-Chlorotoluene 24 October 1, 2010 Cobalt 1 October 1, 2010 Dalapon 200 August 1, 2010 Dibenzofuran 28 May 24, 1999 1,4-Dibromobenzene 70 August 1, 2010 Dibromomethane 70 October 1, 2010 Dichloroacetic Acid 0.7 October 1, 2010 1,2-Dichloroethylene, mixed isomers 60 October 1, 2010 2,4-Dichlorophenol 0.98 August 1, 2010 DDE 0.1 August 1, 2010 Dinoseb 7 August 1, 2010 Diphenyl ether 100 April 1, 2011 2,4-Dinitrotoluene 0.1 April 1, 2011 Diquat 20 August 1, 2010 NORTH CAROLINA ADMINISTRATIVE CODE Eff.