Appendix 8-1: Eelgrass Conservation and Restoration in San Francisco Bay: Opportunities and Constraints
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Section 3.4 Biological Resources 3.4- Biological Resources
SECTION 3.4 BIOLOGICAL RESOURCES 3.4- BIOLOGICAL RESOURCES 3.4 BIOLOGICAL RESOURCES This section discusses the existing sensitive biological resources of the San Francisco Bay Estuary (the Estuary) that could be affected by project-related construction and locally increased levels of boating use, identifies potential impacts to those resources, and recommends mitigation strategies to reduce or eliminate those impacts. The Initial Study for this project identified potentially significant impacts on shorebirds and rafting waterbirds, marine mammals (harbor seals), and wetlands habitats and species. The potential for spread of invasive species also was identified as a possible impact. 3.4.1 BIOLOGICAL RESOURCES SETTING HABITATS WITHIN AND AROUND SAN FRANCISCO ESTUARY The vegetation and wildlife of bayland environments varies among geographic subregions in the bay (Figure 3.4-1), and also with the predominant land uses: urban (commercial, residential, industrial/port), urban/wildland interface, rural, and agricultural. For the purposes of discussion of biological resources, the Estuary is divided into Suisun Bay, San Pablo Bay, Central San Francisco Bay, and South San Francisco Bay (See Figure 3.4-2). The general landscape structure of the Estuary’s vegetation and habitats within the geographic scope of the WT is described below. URBAN SHORELINES Urban shorelines in the San Francisco Estuary are generally formed by artificial fill and structures armored with revetments, seawalls, rip-rap, pilings, and other structures. Waterways and embayments adjacent to urban shores are often dredged. With some important exceptions, tidal wetland vegetation and habitats adjacent to urban shores are often formed on steep slopes, and are relatively recently formed (historic infilled sediment) in narrow strips. -

Dunphy Park / Cass Gidley Marina
1 San Francisco Bay Area Water Trail Site Description for Dunphy Park / Cass Gidley Marina Location, Ownership, and Management: Dunphy Park is a shoreline park owned and managed by the City of Sausalito. The small beach within the park has long served as a popular launch for paddling on Richardson Bay, to Angel Island, and beyond. The historic Cass Gidley Marina is located along the northern side of the park. Dunphy Park is currently undergoing major renovations anticipated to be completed in 2020. Similarly, the Cass Gidley Marina is being redeveloped into the Sausalito Community Boating Center, which will provide access for paddlers and programs for small sailing craft. Contact Name: Mike Langford, Parks and Recreation Director Contact Phone: (415) 289-4126 Contact E-mail: [email protected] Dunphy Park Beach (2019) Dunphy Park Beach (2005) Cass Gidley Marina Facility Description: The non-motorized small boat (NMSB) community has long used the beach at Dunphy Park for launching and landing. Historically, users would park in the unpaved parking area and use adjacent lawn areas to laydown equipment before launching from the small beach. Dunphy Park is currently being redesigned (Fall 2019), which will include improved water access facilities. As part of the Dunphy Park redesign, an ADA ramp and path of travel for beach access will be installed. Steps down to the shoreline will also be provided adjacent to the ramp. Additionally, new ADA restrooms will replace existing portables and parking will be reconfigured to expand capacity and allow better flow. A boat washdown and drinking fountain will also be installed. -
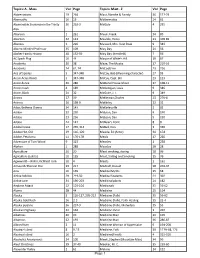
California Folklore Miscellany Index
Topics: A - Mass Vol Page Topics: Mast - Z Vol Page Abbreviations 19 264 Mast, Blanche & Family 36 127-29 Abernathy 16 13 Mathematics 24 62 Abominable Snowman in the Trinity 26 262-3 Mattole 4 295 Alps Abortion 1 261 Mauk, Frank 34 89 Abortion 22 143 Mauldin, Henry 23 378-89 Abscess 1 226 Maxwell, Mrs. Vest Peak 9 343 Absent-Minded Professor 35 109 May Day 21 56 Absher Family History 38 152-59 May Day (Kentfield) 7 56 AC Spark Plug 16 44 Mayor of White's Hill 10 67 Accidents 20 38 Maze, The Mystic 17 210-16 Accidents 24 61, 74 McCool,Finn 23 256 Ace of Spades 5 347-348 McCoy, Bob (Wyoming character) 27 93 Acorn Acres Ranch 5 347-348 McCoy, Capt. Bill 23 123 Acorn dance 36 286 McDonal House Ghost 37 108-11 Acorn mush 4 189 McGettigan, Louis 9 346 Acorn, Black 24 32 McGuire, J. I. 9 349 Acorns 17 39 McKiernan,Charles 23 276-8 Actress 20 198-9 McKinley 22 32 Adair, Bethena Owens 34 143 McKinleyville 2 82 Adobe 22 230 McLean, Dan 9 190 Adobe 23 236 McLean, Dan 9 190 Adobe 24 147 McNear's Point 8 8 Adobe house 17 265, 314 McNeil, Dan 3 336 Adobe Hut, Old 19 116, 120 Meade, Ed (Actor) 34 154 Adobe, Petaluma 11 176-178 Meals 17 266 Adventure of Tom Wood 9 323 Measles 1 238 Afghan 1 288 Measles 20 28 Agriculture 20 20 Meat smoking, storing 28 96 Agriculture (Loleta) 10 135 Meat, Salting and Smoking 15 76 Agwiworld---WWII, Richfield Tank 38 4 Meats 1 161 Aimee McPherson Poe 29 217 Medcalf, Donald 28 203-07 Ainu 16 139 Medical Myths 15 68 Airline folklore 29 219-50 Medical Students 21 302 Airline Lore 34 190-203 Medicinal plants 24 182 Airplane -

SAN FRANCISCO BAY CONSERVATION and DEVELOPMENT COMMISSION May 22, 2007 San Francisco Bay Area Water Trail Steering Committ
SAN FRANCISCO BAY CONSERVATION AND DEVELOPMENT COMMISSION 50 California Street • Suite 2600 • San Francisco, California 94111 • (415) 352-3600 • FAX: (415) 352-3606 • www.bcdc.ca.gov May 22, 2007 TO: San Francisco Bay Area Water Trail Steering Committee and Interested Parties FROM: Sara Polgar, Water Trail Project Manager (415/352-3645 [email protected]) SUBJECT: DRAFT San Francisco Bay Area Water Trail Plan (Comments due by 5:00 p.m., June 19, 2007.) Introduction The enclosed draft Water Trail Plan (Plan) describes policies, guidelines and procedures for implementation of the San Francisco Bay Area Water Trail. Staff is seeking comment on the draft Plan by June 19, 2007, 5:00 p.m. Comments should be submitted to Sara Polgar via email ([email protected]) or mailed to her attention at 50 California Street, Suite 2600, San Francisco, CA 94111. Feedback and corrections to the draft Plan will be incorporated, and a revised draft of the plan will be sent to interested parties in July. Staff will present the revised San Francisco Bay Area Water Trail Plan to the BCDC Commission (July 19, 2007) and the California Coastal Conservancy Board (date TBD). These meetings will be open to the public. For more information about these meetings, visit the BCDC website (www.bcdc.ca.gov) or the Coastal Conservancy website (www.scc.ca.gov). For more information or a hard copy of the draft Plan, contact Sara Polgar. San Francisco Bay Area Water Trail Plan: DRAFT Table of Contents Figures and Tables ..........................................................................................................2 Purpose and Organization of the Plan ........................................................................3 1. -

Latitude 38'S Guide to Bay Sailing
MayCoverTemplate 4/21/09 9:51 AM Page 1 Latitude 38 VOLUME 383 May 2009 WE GO WHERE THE WIND BLOWS MAY 2009 VOLUME 383 BAYGUIDE SAILING TO BAY SAILINGGUIDE Is there anyone out there who's worth of learning the hard way into one and is worth a pass. Stay in the channel not feeling the pinch of the recession? grand tour of the Bay done in style and though, as the northeast side is shallow We doubt it. And yes, many are feeling comfort. We call it the The Perfect Day- and the bottom is riddled with debris. more than a pinch. We're reminded of sail, and it goes like this... Sailing back out the Sausalito Chan- the advice of Thomas Jefferson: "When Start anywhere east of Alcatraz about nel, hug the shoreline and enjoy the you get to the end of your rope, tie a 11 a.m., at which time the fog is begin- Mediterranean look of southern Sau- knot and hang on!" ning to burn off and a light breeze is fill- salito. Generally, the closer you stay to Speaking of ropes and knots and ing in. You're going to be sailing coun- this shore, the flukier the wind — until hanging on, while the 'suits' rage from terclockwise around the Bay, so from you get to Hurricane Gulch. It's not shore while the economy struggles to Alcatraz, head around the backside of marked on the charts, but you'll know extricate itself from the tarpit — we Angel Island and sail west up Raccoon when you're there. -

In This Issue…
Page 1 THE QUIET HAILER October 2010 October 2010 Encouraging education, boating safety, and enjoyment of Sea Ray Boats In this issue. • Officers Reports • Schedule of important events • Bay Cruise report and Photos • Director reports • Upcoming cruise information • Ed and Tech interviews I.B. Wally The QUIET HAILER is a monthly publication of the Sea Ray Boat Club of Northern California Editor: Ernie Macintyre [email protected] Phone(925) 813 0996 Page 2 THE QUIET HAILER October 2010 The Sea Ray Boat Club of Northern California Visit our website at www.srbcnc.org 2010 Calendar of Events Dates Event Location Cruise Leaders January 23-24 Change of Watch Village West Y.C. Roger & Sheila Kelly February 19-21 President's Day Cruise San Joaquin Y.C. Ernie & Dana Macintyre March 20-21 Grinder's Cruise Grindstone Joe's Rick & Judee Myers April 23-25 Asparagus Festival Stocton Waterfront Jason & Heidi Hallenberg May 29-31 Memorial Day American River Rick & Judee Myers May 15 May General meeting Happy Harbour Jim Cupples June 18-20 Mountain William Cruise Locke Slough Kent & Katie Higgins July 3-4 Hilton Fireworks Mandeville Tip Denny & Micki Jensen July 17 July General meeting Willow Berm (Sandy Beach) Jim Cupples August 7-8 Devil's Cruise Little Mandeville Geoge & Linda Overose September 4-6 Circle Cruise Mildred Island Paul & Joanne Sharps October 2-10 Bay Cruise San Francisco Bay Jude & Laura Miller October 29-31 Halloween Cruise Caliente Isle Y.C. Dick & Amelia Wareham November 6 SRBCNC Roast Spindrift Bruce Curley November 26-28 After Thanksgiving Cruise Village West Y.C. -

Lesson 1 18 Focus Question What and Where Is Angel Island
ANGEL ISLAND IMMIGRATION JOURNEYS Lesson 1 LESSON 1: WHERE IS ANGEL ISLAND? AN INTRODUCTORY GEOGRAPHY LESSON Focus Question What and where is Angel Island? Objective Students will reflect on their previous knowledge of immigration. Students will identify the location of Angel Island and its Immigration Station, and create a map with its relation to other geographic features in the San Francisco Bay area. Grades 4 - 8 Time 45 to 50 minute period, plus homework Materials Copies of historical photograph of immigrants arriving at Angel Island to individual students or small groups of students, or Xerox photo on to an overhead transparency and project with an overhead projector on to a wall or screen, Maps such as a map of the United States, San Francisco maps, Angel Island maps, road atlases, Angel Island and California State Parks materials or websites (if a classroom computer is available to the students). Student work sheet with instructions Blank outline map Fine-tip black pens Colored pencils Standards California History-Social Science Standards (See Standards Connections section) Procedure Introduction to Immigration 1. Have students look at photograph of immigrants arriving at Angel Island but passing out photocopies or by projecting the image from an overhead transparency of the photo enlarged on the overhead projector on to a screen or blank wall. 2. Write on the board, the introductory journal entry for the day: “Why might people move from one place to another? Define the word “immigration,” and tell what you know about it.” 3. Have students spend about ten minutes writing on these topics. Have class share ideas. -
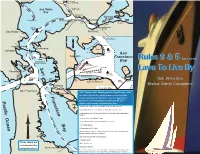
Rule 9 & 5, Laws to Live By
Petaluma River Mare Island Bridge Causeway Bridge Petaluma River Mar e I. Str Vallejo San Pablo ait Channel Bay eet Mare I. Carquinez thball Fl Bridge Mo Dillon Suisun Channel Carqu Pt. Selby inez Bay Pumphouse Davis Pt. Strait Benicia Rodeo Port nole Shoal Crockett Pi Chicago Avon "E"cho Benicia-Martinez Bridge Buoy Pt. Pinole San Rafael Martinez McNears Pt. San Pedro Bluff Pt. Southampton Shoal Channel Pt. San Pablo rait Pt. Simpton Richmond-San Rafael Bridge Richmond Raccoon StAngel I. "A"lpha Larkspur Buoy Red Pt. Knox Rock Long Wharf Pt. Blunt San Sausalito Francisco Rules 9 & 5 ....... Southampton Bay r Shoal Channel Harding e Tiburon t Rock Buoy San Alcatraz I. Deep Wate Westbound Lane Angel I. Traffic Lan Treasure I. Laws To Live By Sausalito Raccoon Strai astbound Lane Berkeley Pier E Blossom Golden Rock Buoy Gate Presidio Shoal Bridge Alcatraz I. Golden Yerba Buena I. Treasure I. Bay Gate San Francisco Bridge Bridge Bay Pt. Bonita San Francisco Bridge Harbor Safety Committee Mile Oakland Rock Central Bay San Francisco Alameda The Captain of the Port designates the following areas (in white) where deep draft commercial and public Francisco vessels routinely operate to be "narrow channels or Pacific Ocean fairways", for the purpose of enforcing RULE 9 (please refter to map for location of sites). OAK • Golden Gate Traffic Lanes and Golden Gate Precautionary Area Hunter's • Central Bay Traffic Lanes and Central Bay Precautionary Area Point • Oakland Harbor Bar Channel and Oakland Outer Harbor and Oakland Inner Harbor San Bruno Shoal • Alameda Naval Air Station Channel • So. -

Deer Discussion Attachment 1 to City Council Staff Report
DEER - ATTACHMENT 1 DEER DISCUSSION ATTACHMENT 1 TO CITY COUNCIL STAFF REPORT STAFF REPORT AND MEMO FROM DEER COMMITTEE NOVEMBER 2009 REPORTS BELVEDERE CITY COUNCIL NOVEMBER 9, 2009 To: Mayor and City Council From: Felicia N. Wheaton, Associate Planner Subject: Findings and recommendations of Deer Committee Recommended Motion/Item Description Review and discuss the findings of the Deer Committee and provide staff with direction with respect to the specific recommendations of the Committee. Background Black-tailed deer are a common sight in Belvedere, particularly on the Island. The deer feed on a variety of plants, traverse well-worn paths, and bed in pockets of dense vegetation. The total number of deer on the Island is unknown, although there is quantity enough to cause aggravation among many of our residents. The City received enough communications expressing concern about the deer to warrant the formation of a citizen committee to research the issues and investigate potential solutions. The Deer Committee held seven public meetings from February to September of 2009. A community-wide questionnaire was conducted to gauge local concern about the issue. The questionnaire had an impressive 50 percent response rate. The majority of respondents resided on the Island and wished for more effort toward deer population control. Concerns ranged from yard damage to fear of personal injury. A summary of the questionnaire results is included in the City of Belvedere Deer Study (Attachment 3). Findings Representatives from the State Department of Fish and Game (DFG) advised the Deer Committee that the deer were a State resource that could not be proactively addressed without the advice and consent of DFG. -

Monthly Reports Executive Board August Cruise Upcoming Events Circle Cruise Bay Cruise Halloween Cruise
August 2017 The Bedrooms Aug 4-6, 2017 In this issue... Monthly Reports Executive Board August Cruise Upcoming Events Circle Cruise Bay Cruise Halloween Cruise Commodore Shelia Kelly Vice Commodore Dave Trowbridge Rear Commodore 2017 Robert Gianelli SRBCNC Secretary Jaime Skehan EXECUTIVE Treasurer OFFICERS Kathy Humphrey The QUIET HAILER more online at www.srbcnc.org p2 more online at www.srbcnc.org The QUIET HAILER 2017 Cruise Schedule Date Cruises & Events Location Leader Crew Jan 28 Change of Watch Stockton YC Kelly Feb 17-19 Mardi Gras Cruise San Joaquin YC Skehan/Trowbridge Shepard, Vinyard March 17-19 Hockey Puck Cruise Stockton Waterfront Murray April 21-23 Fun and Games Cruise Delta YC Lewis Vinyard May 5-7 Mystery Cruise ??? Kelly May 27-29 Memorial Day Cruise Hog Island Palomino June 16-18 Cache Slough Humphrey July 1-4 Independence Day Cruise Mandeville Hand Aug 4-6 Delta Floats Cruise Little Mandeville Palomino Vinyard, Skehan/Trowbridge, Lewis Aug 31-Sept 4 Circle Cruise Mildred Island Humphrey, Trowbridge Palamino, Landcastle, Hand, Vinyard, et al Sept 29-Oct 11 Bay Cruise San Francisco Bay Burger, Humphrey Oct 27-29 Halloween Stockton YC Vinyard Skehan Nov 10-12 Annual SRBCNC Roast Oxbow YC Vinyard Curley 2017 General Meetings (Held at 1:00pm unless otherwise noted) Jan Change of Watch 1/28 (after dinner) Apr Delta YC 4/22 Aug Perry’s 8/26 Oct Stockton YC 10/28 Sea Ray Boat Club of Northern California 2017 Officers & Directors Commodore .................................Shelia Kelly Public Relations ................Scott Landcastle Vice Commodore ............Dave Trowbridge Ed & Tech .....................................Craig Burger Rear Commodore ................Robert Gianelli Programs & Meetings ..............Ken Shepard Immed. -

City of Belvedere Deer Study
City of Belvedere Deer Study BELVEDERE, MARIN COUNTY, CALIFORNIA Prepared For: City of Belvedere Deer Committee 450 San Rafael Avenue Belvedere, California 94920 Contact: Jeff Dreier [email protected] Date: June 2009 TABLE OF CONTENTS Introduction ....................................................................................................................... 2 Black-tailed Deer Life History............................................................................................ 2 Study Area ........................................................................................................................ 3 Habitat...........................................................................................................................3 Deer Population ............................................................................................................3 Methods ............................................................................................................................ 5 Questionnaire................................................................................................................5 Literature Review ..........................................................................................................5 Results .............................................................................................................................. 5 Questionnaire................................................................................................................5 Opinions Regarding Deer..........................................................................................5 -

Feb 2016 Seascape
FEBRUARY 2016 Commodore Donna Beckett (510) 857-6599 – [email protected] I am very happy to welcome our new Food and Beverage manager, Theresa Barnes. Theresa will be joining us on February 1. Her duties will be to manage the galley staff, make sure the galley runs smoothly and members enjoy a wonderful dining experience. With her coming on board Adam will now be in the office full time as the office manager. As you’re reading this you are probably aware by now that the San Francisco Progressive Boat show has been cancelled. Because of the last change in date due to storm damage, many of the exhibitors were not able to make the new date and so the show was called off. The Richmond Strictly Sail show is still on and we will have a booth there. Volunteers are needed to work the show. It only takes a few hours of your time, you get to meet some interesting people, it’s a great help to the Club and you get free admittance to the show. Volunteer and help build membership. Our Valentine’s Day party will be a very special evening. Chef Matt has planned a gourmet menu. We have a very popular musician scheduled who had everyone at the Change of Watch out of their chairs and on the dance floor. So plan to take your sweetie to the Club for a wonderful and memorable evening of dining and dancing. The Club is looking to move away from accepting cash at the bar and the galley.