DRUG NAME: Cyclophosphamide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
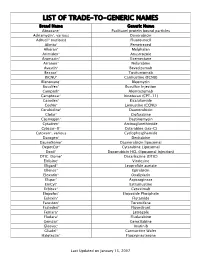
Trade-To-Generic Names
LIST OF TRADE-TO-GENERIC NAMES Brand Name Generic Name Abraxane® Paclitaxel protein bound particles Adriamycin®, various Doxorubicin Adrucil® (various) Fluorouracil Alimta® Pemetrexed Alkeran® Melphalan Arimidex® Anastrozole Aromasin® Exemestane Arranon® Nelarabine Avastin® Bevacizumab Bexxar® Tositumomab BiCNU® Carmustine (BCNU) Blenoxane® Bleomycin Busulfex® Busulfan Injection Campath® Alemtuzumab Camptosar® Irinotecan (CPT-11) Casodex® Bicalutamide CeeNu® Lomustine (CCNU) Cerubidine® Daunorubicin Clolar® Clofarabine Cosmegen® Dactinomycin Cytadren® Aminoglutethimide Cytosar-U® Cytarabine (ara-C) Cytoxan®, various Cyclophosphamide Dacogen® Decitabine DaunoXome® Daunorubicin liposomal DepotCyt® Cytarabine Liposomal Doxil® Doxorubicin HCL (liposomal injection) DTIC-Dome® Dacarbazine (DTIC) Eldisine® Vindesine Eligard® Leuprolide acetate Ellence® Epirubicin Eloxatin® Oxaliplatin Elspar® Asparaginase EmCyt® Estramustine Erbitux® Cetuximab Etopofos® Etoposide Phosphate Eulexin® Flutamide Fareston® Toremifene Faslodex® Fluvestrant Femara® Letrozole Fludara® Fludarabine Gemzar® Gemcitabine Gleevec® Imatinib Gliadel® Carmustine Wafer Halotestin® Fluoxymesterone Last Updated on January 15, 2007 Brand Name Generic Name Herceptin® Trastuzumab Hexalen® Altretamine Hycamtin® Topotecan Hydrea® Hydroxyurea Idamycin® Idarubicin Ifex® Ifosfamide Intron A® Interferon alfa-2b Iressa® Gefitinib Leukeran® Chlorambucil Leukine® Sargramostim Leustatin® Cladribine Lupron depot® Leuprolide acetate depot Lupron® Leuprolide acetate Matulane® Procarbazine Megace® -

Cyclophosphamide-Etoposide PO Ver
Chemotherapy Protocol LYMPHOMA CYCLOPHOSPHAMIDE-ETOPOSIDE ORAL Regimen Lymphoma – Cyclophosphamide-Etoposide PO Indication Palliative treatment of malignant lymphoma Toxicity Drug Adverse Effect Cyclophosphamide Dysuria, haemorrragic cystitis (rare), taste disturbances Etoposide Alopecia, hyperbilirubinaemia The adverse effects listed are not exhaustive. Please refer to the relevant Summary of Product Characteristics for full details. Patients diagnosed with Hodgkin’s Lymphoma carry a lifelong risk of transfusion associated graft versus host disease (TA-GVHD). Where blood products are required these patients must receive only irradiated blood products for life. Local blood transfusion departments must be notified as soon as a diagnosis is made and the patient must be issued with an alert card to carry with them at all times. Monitoring Drugs FBC, LFTs and U&Es prior to day one of treatment Albumin prior to each cycle Dose Modifications The dose modifications listed are for haematological, liver and renal function and drug specific toxicities only. Dose adjustments may be necessary for other toxicities as well. In principle all dose reductions due to adverse drug reactions should not be re-escalated in subsequent cycles without consultant approval. It is also a general rule for chemotherapy that if a third dose reduction is necessary treatment should be stopped. Please discuss all dose reductions / delays with the relevant consultant before prescribing, if appropriate. The approach may be different depending on the clinical circumstances. Version 1.1 (Jan 2015) Page 1 of 6 Lymphoma- Cyclophosphamide-Etoposide PO Haematological Dose modifications for haematological toxicity in the table below are for general guidance only. Always refer to the responsible consultant as any dose reductions or delays will be dependent on clinical circumstances and treatment intent. -

PEMD-91-12BR Off-Label Drugs: Initial Results of a National Survey
11 1; -- __...._-----. ^.-- ______ -..._._ _.__ - _........ - t Ji Jo United States General Accounting Office Washington, D.C. 20648 Program Evaluation and Methodology Division B-242851 February 25,199l The Honorable Edward M. Kennedy Chairman, Committee on Labor and Human Resources United States Senate Dear Mr. Chairman: In September 1989, you asked us to conduct a study on reimbursement denials by health insurers for off-label drug use. As you know, the Food and Drug Administration designates the specific clinical indications for which a drug has been proven effective on a label insert for each approved drug, “Off-label” drug use occurs when physicians prescribe a drug for clinical indications other than those listed on the label. In response to your request, we surveyed a nationally representative sample of oncologists to determine: . the prevalence of off-label use of anticancer drugs by oncologists and how use varies by clinical, demographic, and geographic factors; l the extent to which third-party payers (for example, Medicare intermediaries, private health insurers) are denying payment for such use; and l whether the policies of third-party payers are influencing the treatment of cancer patients. We randomly selected 1,470 members of the American Society of Clinical Oncologists and sent them our survey in March 1990. The sam- pling was structured to ensure that our results would be generalizable both to the nation and to the 11 states with the largest number of oncologists. Our response rate was 56 percent, and a comparison of respondents to nonrespondents shows no noteworthy differences between the two groups. -

Inhibition of Cyclophosphamide and Mitomycin C-Induced Sister Chromatid Exchanges in Mice by Vitamin C
ICANCERRESEARCH46,2670-2674, June 19861 Inhibition of Cyclophosphamide and Mitomycin C-induced Sister Chromatid Exchanges in Mice by Vitamin C G. Krishna,' J. Nath, and T. Ong Natio,wilnstitutefor OccupationaiSafety and Health, Division ofRespiratory Disease Studies, Morgansown, West Virginia 26505-2888 fG. K., T. 0.], andDivision of PlantandSoil Sciences,West VirginiaUniversity,Morgantown,West Visijnia 265O6fJ.N.J ABSTRACF The research reported here was performed to determine the effect of ascorbic acid on SCEs induced by CPA and MMC in Ascorbic acid (vitamin C) is known to act as an antimutagen and in vivo and in vivo/in vitro conditions in bone marrow and anticarcinogen in several test systems. However, there is no report of its spleen cells of mice. Analysis of SCEs is a sensitive cytogenetic effect on carcinogen-induced chromosomal damage in vii'o in animals. technique for detecting cellular chromosomal damage (11). The present study was performed to determine whether or not ascorbic SCEs are visualized as reciprocal exchangesof staining inten acid affects sister ébromatidexchanges (SCEs) induced by cyclophos sities between sister chromatid arms in metaphase cells that phamide (CPA) and mitomycin C (MMC) in bone marrow and spleen cells in mice. The results indicate that ascorbic acid per se did not cause have replicated twice in the presenceof BrdUrd. a significantincreaseinSCEsin mice.However,increasingconcentra tions of ascorbic acid caused decreasing levels of CPA- and MMC induced SCEs in both cell types in rho. At the highest concentration of MATERIALS AND METHODS ascorbic acid, 6.68 gfkg, approximately 75 and 40% SCE inhibition in Animals. Male CD-I mice were purchased from Charles River Breed both cell types was noted for CPA and MMC, respectively. -

AHFS Pharmacologic-Therapeutic Classification System
AHFS Pharmacologic-Therapeutic Classification System Abacavir 48:24 - Mucolytic Agents - 382638 8:18.08.20 - HIV Nucleoside and Nucleotide Reverse Acitretin 84:92 - Skin and Mucous Membrane Agents, Abaloparatide 68:24.08 - Parathyroid Agents - 317036 Aclidinium Abatacept 12:08.08 - Antimuscarinics/Antispasmodics - 313022 92:36 - Disease-modifying Antirheumatic Drugs - Acrivastine 92:20 - Immunomodulatory Agents - 306003 4:08 - Second Generation Antihistamines - 394040 Abciximab 48:04.08 - Second Generation Antihistamines - 394040 20:12.18 - Platelet-aggregation Inhibitors - 395014 Acyclovir Abemaciclib 8:18.32 - Nucleosides and Nucleotides - 381045 10:00 - Antineoplastic Agents - 317058 84:04.06 - Antivirals - 381036 Abiraterone Adalimumab; -adaz 10:00 - Antineoplastic Agents - 311027 92:36 - Disease-modifying Antirheumatic Drugs - AbobotulinumtoxinA 56:92 - GI Drugs, Miscellaneous - 302046 92:20 - Immunomodulatory Agents - 302046 92:92 - Other Miscellaneous Therapeutic Agents - 12:20.92 - Skeletal Muscle Relaxants, Miscellaneous - Adapalene 84:92 - Skin and Mucous Membrane Agents, Acalabrutinib 10:00 - Antineoplastic Agents - 317059 Adefovir Acamprosate 8:18.32 - Nucleosides and Nucleotides - 302036 28:92 - Central Nervous System Agents, Adenosine 24:04.04.24 - Class IV Antiarrhythmics - 304010 Acarbose Adenovirus Vaccine Live Oral 68:20.02 - alpha-Glucosidase Inhibitors - 396015 80:12 - Vaccines - 315016 Acebutolol Ado-Trastuzumab 24:24 - beta-Adrenergic Blocking Agents - 387003 10:00 - Antineoplastic Agents - 313041 12:16.08.08 - Selective -

BC Cancer Protocol Summary for Treatment of Lymphoma with Dose- Adjusted Etoposide, Doxorubicin, Vincristine, Cyclophosphamide
BC Cancer Protocol Summary for Treatment of Lymphoma with Dose- Adjusted Etoposide, DOXOrubicin, vinCRIStine, Cyclophosphamide, predniSONE and riTUXimab with Intrathecal Methotrexate Protocol Code LYEPOCHR Tumour Group Lymphoma Contact Physician Dr. Laurie Sehn Dr. Kerry Savage ELIGIBILITY: One of the following lymphomas: . Patients with an aggressive B-cell lymphoma and the presence of a dual translocation of MYC and BCL2 (i.e., double-hit lymphoma). Histologies may include DLBCL, transformed lymphoma, unclassifiable lymphoma, and intermediate grade lymphoma, not otherwise specified (NOS). Patients with Burkitt lymphoma, who are not candidates for CODOXM/IVACR (such as those over the age of 65 years, or with significant co-morbidities) . Primary mediastinal B-cell lymphoma Ensure patient has central line EXCLUSIONS: . Cardiac dysfunction that would preclude the use of an anthracycline. TESTS: . Baseline (required before first treatment): CBC and diff, platelets, BUN, creatinine, bilirubin. ALT, LDH, uric acid . Baseline (required, but results do not have to be available to proceed with first treatment): results must be checked before proceeding with cycle 2): HBsAg, HBcoreAb, . Baseline (optional, results do not have to be available to proceed with first treatment): HCAb, HIV . Day 1 of each cycle: CBC and diff, platelets, (and serum bilirubin if elevated at baseline; serum bilirubin does not need to be requested before each treatment, after it has returned to normal), urinalysis for microscopic hematuria (optional) . Days 2 and 5 of each cycle (or days of intrathecal treatment): CBC and diff, platelets, PTT, INR . For patients on cyclophosphamide doses greater than 2000 mg: Daily urine dipstick for blood starting on day cyclophosphamide is given. -

Arsenic Trioxide Is Highly Cytotoxic to Small Cell Lung Carcinoma Cells
160 Arsenic trioxide is highly cytotoxic to small cell lung carcinoma cells 1 1 Helen M. Pettersson, Alexander Pietras, effect of As2O3 on SCLC growth, as suggested by an Matilda Munksgaard Persson,1 Jenny Karlsson,1 increase in neuroendocrine markers in cultured cells. [Mol Leif Johansson,2 Maria C. Shoshan,3 Cancer Ther 2009;8(1):160–70] and Sven Pa˚hlman1 1Center for Molecular Pathology, CREATE Health and 2Division of Introduction Pathology, Department of Laboratory Medicine, Lund University, 3 Lung cancer is the most frequent cause of cancer deaths University Hospital MAS, Malmo¨, Sweden; and Department of f Oncology-Pathology, Cancer Center Karolinska, Karolinska worldwide and results in 1 million deaths each year (1). Institute and Hospital, Stockholm, Sweden Despite novel treatment strategies, the 5-year survival rate of lung cancer patients is only f15%. Small cell lung carcinoma (SCLC) accounts for 15% to 20% of all lung Abstract cancers diagnosed and is a very aggressive malignancy Small cell lung carcinoma (SCLC) is an extremely with early metastatic spread (2). Despite an initially high aggressive form of cancer and current treatment protocols rate of response to chemotherapy, which currently com- are insufficient. SCLC have neuroendocrine characteristics bines a platinum-based drug with another cytotoxic drug and show phenotypical similarities to the childhood tumor (3, 4), relapses occur in the absolute majority of SCLC neuroblastoma. As multidrug-resistant neuroblastoma patients. At relapse, the efficacy of further chemotherapy is cells are highly sensitive to arsenic trioxide (As2O3) poor and the need for alternative treatments is obvious. in vitro and in vivo, we here studied the cytotoxic effects Arsenic-containing compounds have been used in tradi- of As2O3 on SCLC cells. -

Chemotherapy Protocol
Chemotherapy Protocol BREAST CANCER CYCLOPHOSPHAMIDE-EPIRUBICIN-PACLITAXEL (7 day) Regimen • Breast Cancer – Cyclophosphamide-Epirubicin-Paclitaxel (7 day) Indication • Neoadjuvant / adjuvant therapy of early breast cancer • WHO Performance status 0, 1 Toxicity Drug Adverse Effect Cyclophosphamide Dysuria, haemorrhagic cystitis, taste disturbances Epirubicin Cardio-toxicity, urinary discolouration (red) Hypersensitivity, hypotension, bradycardia, peripheral Paclitaxel neuropathy, myalgia and back pain on administration The adverse effects listed are not exhaustive. Please refer to the relevant Summary of Product Characteristics for full details. Monitoring Regimen • FBC, U&Es and LFTs prior to each cycle. • Ensure adequate cardiac function before starting treatment with epirubicin. Baseline LVEF should be measured, particularly in patients with a history of cardiac problems or in the elderly. Dose Modifications The dose modifications listed are for haematological, liver and renal function only. Dose adjustments may be necessary for other toxicities as well. In principle all dose reductions due to adverse drug reactions should not be re- escalated in subsequent cycles without consultant approval. It is also a general rule for chemotherapy that if a third dose reduction is necessary treatment should be stopped. Please discuss all dose reductions / delays with the relevant consultant before prescribing if appropriate. The approach may be different depending on the clinical circumstances. The following is a general guide only. Version 1.2 (November 2020) Page 1 of 7 Breast – Cyclophosphamide-Epirubicin-Paclitaxel (7 day) Haematological Prior to prescribing the following treatment criteria must be met on day one of treatment. Criteria Eligible Level Neutrophils equal to or more than 1x10 9/L Platelets equal to or more than 100x10 9/L Consider blood transfusion if patient symptomatic of anaemia or has a haemoglobin of less than 8g/dL If counts on day one are below these criteria for neutrophils and platelets then delay treatment for seven days. -

Arsenic Trioxide Potentiates the Effectiveness of Etoposide in Ewing Sarcomas
INTERNATIONAL JOURNAL OF ONCOLOGY 49: 2135-2146, 2016 Arsenic trioxide potentiates the effectiveness of etoposide in Ewing sarcomas KAREN A. BOEHME1*, JULIANE NITSCH1*, ROSA RIESTER1, RUPert HANDGRETINGER2, SABINE B. SCHLEICHER2, TORSTEN KLUBA3,4 and FRANK TRAUB1,3 1Laboratory of Cell Biology, Department of Orthopaedic Surgery, Eberhard Karls University Tuebingen; 2Department of Haematology and Oncology, Children's Hospital, Eberhard Karls University Tuebingen; 3Department of Orthopaedic Surgery, Eberhard Karls University Tuebingen, Tuebingen; 4Department for Orthopaedic Surgery, Hospital Dresden-Friedrichstadt, Dresden, Germany Received June 12, 2016; Accepted July 28, 2016 DOI: 10.3892/ijo.2016.3700 Abstract. Ewing sarcomas (ES) are rare mesenchymal tumours, the drug concentrations used. With the exception of ATO in most commonly diagnosed in children and adolescents. Arsenic RD-ES cells, all drugs induced apoptosis in the ES cell lines, trioxide (ATO) has been shown to efficiently and selectively indicated by caspase-3 and PARP cleavage. Combination of target leukaemic blasts as well as solid tumour cells. Since the agents potentiated the reduction of viability as well as the multidrug resistance often occurs in recurrent and metastatic inhibitory effect on clonal growth. In addition, cell death induc- ES, we tested potential additive effects of ATO in combina- tion was obviously enhanced in RD-ES and SK-N-MC cells tion with the cytostatic drugs etoposide and doxorubicin. The by a combination of ATO and etoposide compared to single Ewing sarcoma cell lines A673, RD-ES and SK-N-MC as well application. Summarised, the combination of low dose, physi- as mesenchymal stem cells (MSC) for control were treated ologically easily tolerable ATO with commonly used etoposide with ATO, etoposide and doxorubicin in single and combined and doxorubicin concentrations efficiently and selectively application. -

5-Fluorouracil + Adriamycin + Cyclophosphamide) Combination in Differentiated H9c2 Cells
Article Doxorubicin Is Key for the Cardiotoxicity of FAC (5-Fluorouracil + Adriamycin + Cyclophosphamide) Combination in Differentiated H9c2 Cells Maria Pereira-Oliveira, Ana Reis-Mendes, Félix Carvalho, Fernando Remião, Maria de Lourdes Bastos and Vera Marisa Costa * UCIBIO, REQUIMTE, Laboratory of Toxicology, Faculty of Pharmacy, University of Porto, Rua de Jorge Viterbo Ferreira, 228, 4050-313 Porto, Portugal; [email protected] (M.P.-O.); [email protected] (A.R.-M.); [email protected] (F.C.); [email protected] (F.R.); [email protected] (M.L.B.) * Correspondence: [email protected] Received: 4 October 2018; Accepted: 3 January 2019; Published: 10 January 2019 Abstract: Currently, a common therapeutic approach in cancer treatment encompasses a drug combination to attain an overall better efficacy. Unfortunately, it leads to a higher incidence of severe side effects, namely cardiotoxicity. This work aimed to assess the cytotoxicity of doxorubicin (DOX, also known as Adriamycin), 5-fluorouracil (5-FU), cyclophosphamide (CYA), and their combination (5-Fluorouracil + Adriamycin + Cyclophosphamide, FAC) in H9c2 cardiac cells, for a better understanding of the contribution of each drug to FAC-induced cardiotoxicity. Differentiated H9c2 cells were exposed to pharmacological relevant concentrations of DOX (0.13–5 μM), 5-FU (0.13–5 μM), CYA (0.13–5 μM) for 24 or 48 h. Cells were also exposed to FAC mixtures (0.2, 1 or 5 μM of each drug and 50 μM 5-FU + 1 μM DOX + 50 μM CYA). DOX was the most cytotoxic drug, followed by 5-FU and lastly CYA in both cytotoxicity assays (reduction of 3-(4,5-dimethylthiazol-2- yl)-2,5-diphenyl tetrazolium bromide (MTT) and neutral red (NR) uptake). -

Rasburicase (Elitek)
Rasburicase (Elitek) ELITEK™ (rasburicase) BOXED WARNINGS Anaphylaxis ELITEK may cause severe hypersensitivity reactions including anaphylaxis. ELITEK should be immediately and permanently discontinued in any patient developing clinical evidence of a serious hypersensitivity reaction (see WARNINGS, Anaphylaxis and ADVERSE REACTIONS, Immunogenicity). Hemolysis ELITEK administered to patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency can cause severe hemolysis. ELITEK administration should be immediately and permanently discontinued in any patient developing hemolysis. It is recommended that patients at higher risk for G6PD deficiency (e.g., patients of African or Mediterranean ancestry) be screened prior to starting ELITEK therapy (see CONTRAINDICATIONS and WARNINGS, Hemolysis). Methemoglobinemia ELITEK use has been associated with Methemoglobinemia. ELITEK administration should be immediately and permanently discontinued in any patient identified as having developed methemoglobinemia (see WARNINGS, Methemoglobinemia). Interference with Uric Acid Measurements ELITEK will cause enzymatic degradation of the uric acid within blood samples left at room temperature, resulting in spuriously low uric acid levels. To ensure accurate measurements, blood must be collected into pre-chilled tubes containing heparin anticoagulent and immediately immersed and maintained in an ice water bath; plasma samples must be assayed within 4 hours of sample collection (see PRECAUTIONS, Laboratory Test Interactions). DESCRIPTION ELITEK (rasburicase) is a recombinant urate-oxidase enzyme produced by a genetically modified Saccharomyces cerevisiae strain. The cDNA coding for rasburicase was cloned from a strain of Aspergillus flavus. Rasburicase is a tetrameric protein with identical subunits of a molecular mass of about 34 kDa. The molecular formula of the monomer is C1523 H2383 N417 O462 S7. The monomer, made up of a single 301 amino acid polypeptide chain, has no intra- or inter-disulfide bridges and is N-terminal acetylated. -

New Brunswick Prescription Drug Program Formulary
NEW BRUNSWICK PRESCRIPTION DRUG PROGRAM FORMULARY Copyright - 2003 The Queen In the right of The Province of New Brunswick as represented by The Honourable Elvy Robichaud Minister of Health and Wellness ADMINISTERED BY ATLANTIC BLUE CROSS CARE ON BEHALF OF THE GOVERNMENT OF NEW BRUNSWICK TABLE OF CONTENTS Page Introduction to the New Brunswick Prescription Drug Program Formulary I The New Brunswick Prescription Drug Program I New Brunswick Prescription Drug Program Plans II - III Exclusions IV - V Extemporaneous Preparations and Placebos VI Benefit Review Process VII Product Deletion VII Submission Requirements VIII Legend IX Comment Sheet X Pharmacologic - Therapeutic Classification of Drugs 4:00 Antihistamines 1 8:00 Anti-Infective Agents 3 10:00 Antineoplastic Agents 28 12:00 Autonomic Drugs 33 20:00 Blood Formation and Coagulation 43 24:00 Cardiovascular Agents 49 28:00 Central Nervous System Agent 76 40:00 Electrolytic, Caloric and Water Balance 128 48:00 Antitussives, Expectorants & Mucolytic Agents 133 52:00 Eye, Ear, Nose and Throat (EENT) Preparations 135 56:00 Gastrointestinal Drugs 149 60:00 Gold Compounds 159 64:00 Heavy Metal Antagonists 160 68:00 Hormones and Synthetic Substitutes 161 80:00 Serums, Toxoids, and Vaccines 179 84:00 Skin and Mucous Membrane Agents 180 86:00 Smooth Muscle Relaxants 198 88:00 Vitamins 201 92:00 Unclassified Therapeutic Agents 205 TABLE OF CONTENTS Page Appendices I-A Abbreviations of Dosage Forms A-1 - A-3 I-B Abbreviations of Routes A-4 - A-5 I-C Abbreviations of Units A-6 - A-7 II Abbreviations of Manufacturers' Names A-8 - A-9 III Extemporaneous Preparations A-10 IV Special Authorization A-11 IV Special Authorization Drug Criteria A-12 NEW BRUNSWICK PRESCRIPTION DRUG PROGRAM FORMULARY Introduction The New Brunswick Prescription Drug Program Formulary is a listing of all drugs and drug products which have been determined by the Minister of Health and Wellness to be entitled services.