Embryology and Development: Mandible, Maxillary, Deciduous and Permanent Teeth
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Is the Skeleton Male Or Female? the Pelvis Tells the Story
Activity: Is the Skeleton Male or Female? The pelvis tells the story. Distinct features adapted for childbearing distinguish adult females from males. Other bones and the skull also have features that can indicate sex, though less reliably. In young children, these sex-related features are less obvious and more difficult to interpret. Subtle sex differences are detectable in younger skeletons, but they become more defined following puberty and sexual maturation. What are the differences? Compare the two illustrations below in Figure 1. Female Pelvic Bones Male Pelvic Bones Broader sciatic notch Narrower sciatic notch Raised auricular surface Flat auricular surface Figure 1. Female and male pelvic bones. (Source: Smithsonian Institution, illustrated by Diana Marques) Figure 2. Pelvic bone of the skeleton in the cellar. (Source: Smithsonian Institution) Skull (Cranium and Mandible) Male Skulls Generally larger than female Larger projections behind the Larger brow ridges, with sloping, ears (mastoid processes) less rounded forehead Square chin with a more vertical Greater definition of muscle (acute) angle of the jaw attachment areas on the back of the head Figure 3. Male skulls. (Source: Smithsonian Institution, illustrated by Diana Marques) Female Skulls Smoother bone surfaces where Smaller projections behind the muscles attach ears (mastoid processes) Less pronounced brow ridges, Chin more pointed, with a larger, with more vertical forehead obtuse angle of the jaw Sharp upper margins of the eye orbits Figure 4. Female skulls. (Source: Smithsonian Institution, illustrated by Diana Marques) What Do You Think? Comparing the skull from the cellar in Figure 5 (below) with the illustrated male and female skulls in Figures 3 and 4, write Male or Female to note the sex depicted by each feature. -

The Cat Mandible (II): Manipulation of the Jaw, with a New Prosthesis Proposal, to Avoid Iatrogenic Complications
animals Review The Cat Mandible (II): Manipulation of the Jaw, with a New Prosthesis Proposal, to Avoid Iatrogenic Complications Matilde Lombardero 1,*,† , Mario López-Lombardero 2,†, Diana Alonso-Peñarando 3,4 and María del Mar Yllera 1 1 Unit of Veterinary Anatomy and Embryology, Department of Anatomy, Animal Production and Clinical Veterinary Sciences, Faculty of Veterinary Sciences, Campus of Lugo—University of Santiago de Compostela, 27002 Lugo, Spain; [email protected] 2 Engineering Polytechnic School of Gijón, University of Oviedo, 33203 Gijón, Spain; [email protected] 3 Department of Animal Pathology, Faculty of Veterinary Sciences, Campus of Lugo—University of Santiago de Compostela, 27002 Lugo, Spain; [email protected] 4 Veterinary Clinic Villaluenga, calle Centro n◦ 2, Villaluenga de la Sagra, 45520 Toledo, Spain * Correspondence: [email protected]; Tel.: +34-982-822-333 † Both authors contributed equally to this manuscript. Simple Summary: The small size of the feline mandible makes its manipulation difficult when fixing dislocations of the temporomandibular joint or mandibular fractures. In both cases, non-invasive techniques should be considered first. When not possible, fracture repair with internal fixation using bone plates would be the best option. Simple jaw fractures should be repaired first, and caudal to rostral. In addition, a ventral approach makes the bone fragments exposure and its manipulation easier. However, the cat mandible has little space to safely place the bone plate screws without damaging the tooth roots and/or the mandibular blood and nervous supply. As a consequence, we propose a conceptual model of a mandibular prosthesis that would provide biomechanical Citation: Lombardero, M.; stabilization, avoiding any unintended (iatrogenic) damage to those structures. -

Results Description of the SKULLS. the Overall Size of Both Skulls Was Considered to Be Within Normal Limits for Their Ethnic
Ossification Defects and Craniofacial Morphology In Incomplete Forms of Mandibulofacial Dysostosis A Description of Two Dry Skulls ERIK DAHL, D.D.S., DR. ODONT. ARNE BJORK, D.D.S., ODONT. DR. Copenhagen, Denmark The morphology of two East Indian dry skulls exhibiting anomalies which were suggested to represent incomplete forms of mandibulofacial dysostosis is described. Obvious although minor ossification anomalies were found localized to the temporal, sphenoid, the zygomatic, the maxillary and the mandibular bones. The observations substantiate the concept of the regional and bilateral nature of this malformation syndrome. Bilateral orbital deviations, hypoplasia of the malar bones, and incomplete zygomatic arches appear to be hard tissue aberrations which may be helpful in exami- nation for subclinical carrier status. Changes in mandibular morphology seem to be less distinguishing features in incomplete or abortive types of mandibulofacial dysostosis. KEY WORDS craniofacial problems, mandible, mandibulofacial dysostosis, maxilla, sphenoid bone, temporal bone, zygomatic bone Mandibulofacial dysostosis (MFD) often roentgencephalometric examinations were results in the development of a characteristic made of the skulls, and tomograms were ob- facial disfigurement with considerable simi- tained of the internal and middle ear. Com- larity between affected individuals. However, parisons were made with normal adult skulls the symptoms may vary highly in respect to and with an adult skull exhibiting the char- type and degree, and both incomplete and acteristics of MFD. All of the skulls were from abortive forms of the syndrome have been the same ethnic group. ' reported in the literature (Franceschetti and Klein, 1949; Moss et al., 1964; Rogers, 1964). Results In previous papers, we have shown the DEsCRIPTION OF THE SKULLS. -

GLOSSARY of MEDICAL and ANATOMICAL TERMS
GLOSSARY of MEDICAL and ANATOMICAL TERMS Abbreviations: • A. Arabic • abb. = abbreviation • c. circa = about • F. French • adj. adjective • G. Greek • Ge. German • cf. compare • L. Latin • dim. = diminutive • OF. Old French • ( ) plural form in brackets A-band abb. of anisotropic band G. anisos = unequal + tropos = turning; meaning having not equal properties in every direction; transverse bands in living skeletal muscle which rotate the plane of polarised light, cf. I-band. Abbé, Ernst. 1840-1905. German physicist; mathematical analysis of optics as a basis for constructing better microscopes; devised oil immersion lens; Abbé condenser. absorption L. absorbere = to suck up. acervulus L. = sand, gritty; brain sand (cf. psammoma body). acetylcholine an ester of choline found in many tissue, synapses & neuromuscular junctions, where it is a neural transmitter. acetylcholinesterase enzyme at motor end-plate responsible for rapid destruction of acetylcholine, a neurotransmitter. acidophilic adj. L. acidus = sour + G. philein = to love; affinity for an acidic dye, such as eosin staining cytoplasmic proteins. acinus (-i) L. = a juicy berry, a grape; applied to small, rounded terminal secretory units of compound exocrine glands that have a small lumen (adj. acinar). acrosome G. akron = extremity + soma = body; head of spermatozoon. actin polymer protein filament found in the intracellular cytoskeleton, particularly in the thin (I-) bands of striated muscle. adenohypophysis G. ade = an acorn + hypophyses = an undergrowth; anterior lobe of hypophysis (cf. pituitary). adenoid G. " + -oeides = in form of; in the form of a gland, glandular; the pharyngeal tonsil. adipocyte L. adeps = fat (of an animal) + G. kytos = a container; cells responsible for storage and metabolism of lipids, found in white fat and brown fat. -

Paramedian Mandibular Cleft in a Patient Who Also Had Goldenhar 2
Brief Clinical Studies The Journal of Craniofacial Surgery & Volume 23, Number 1, January 2012 as the thyroid gland and hyoid bone, to determine whether any 10. Franzese C, Hayes JD, Nichols K. Congenital midline cervical cleft: a associated anomalies exist.3,16 Alternatively, CT or magnetic reso- report of two cases. Ear Nose Throat J 2008;87:166Y168 nance imaging may be performed for a more thorough assessment 11. Hirokawa S, Uotani H, Okami H, et al. A case of congenital midline of the soft tissue relationships; in our case, a CT scan of the neck cervical cleft with congenital heart disease. J Pediatr Surg Y confirmed a superficial subcutaneous cord, without deeper tissue 2003;38:1099 1101 involvement. To determine the source of airway obstruction, pre- 12. Tsukuno M, Kita Y, Kurihara K. A case of midline cervical cleft. Congenit Anom (Kyoto) 2002;42:143Y145 operative flexible laryngoscopy should be performed. 13. Vure S, Pang K, Hallam L, et al. Congenital midline cervical cleft Surgical treatment of CMCC is required to alleviate or prevent with an underlying bronchogenic like cyst. Pediatr Surg Int anterior neck contracture, respiratory distress, micrognathia, and 2009;25:811Y813 4,5,13 infection and for aesthetic reasons. Treatment involves the com- 14. Andryk JE, Kerschner JE, Hung RT, et al. Mid-line cervical cleft with a plete excision of the lesion and any involved tissues, followed by bronchogenic cyst. Int J Pediatr Otorhinolaryngol 1999;47:261Y264 closure, which is most commonly performed with a Z-plasty or mul- 15. Agag R, Sacks J, Silver L. -

Sensitive Teeth Sensitive Teeth Can Be Treated
FOR THE DENTAL PATIENT ... TREATMENT Sensitive teeth Sensitive teeth can be treated. Depending on the cause, your dentist may suggest that you try Causes and treatment desensitizing toothpaste, which contains com- pounds that help block sensation traveling from the tooth surface to the nerve. Desensitizing f a taste of ice cream or a sip of coffee is toothpaste usually requires several applications sometimes painful or if brushing or flossing before the sensitivity is reduced. When choosing makes you wince occasionally, you may toothpaste or any other dental care products, look have a common problem called “sensitive for those that display the American Dental Asso- teeth.” Some of the causes include tooth ciation’s Seal of Acceptance—your assurance that Idecay, a cracked tooth, worn tooth enamel, worn products have met ADA criteria for safety and fillings and tooth roots that are exposed as a effectiveness. result of aggressive tooth brushing, gum recession If the desensitizing toothpaste does not ease and periodontal (gum) disease. your discomfort, your dentist may suggest in- office treatments. A fluoride gel or special desen- SYMPTOMS OF SENSITIVE TEETH sitizing agents may be applied to the sensitive A layer of enamel, the strongest substance in the areas of the affected teeth. When these measures body, protects the crowns of healthy teeth. A layer do not correct the problem, your dentist may rec- called cementum protects the tooth root under the ommend other treatments, such as a filling, a gum line. Underneath the enamel and the crown, an inlay or bonding to correct a flaw or cementum is dentin, a part of the tooth that is decay that results in sensitivity. -

Original Article Concurrent Measurements of Danger Zone Anatomy in Mandibular First Molars Using Micro-Computed Tomography: a Pilot Study
Int J Clin Exp Med 2018;11(8):7692-7700 www.ijcem.com /ISSN:1940-5901/IJCEM0067321 Original Article Concurrent measurements of danger zone anatomy in mandibular first molars using micro-computed tomography: a pilot study Jiawei Lu1, Lizong Liang1, Jie Ran1, Gao Wu1, Chenghao Li2, Jianping Ge2, Jianxiang Tao1 Departments of 1Prosthodontics, 2Endodontics, School and Hospital of Stomatology, Tongji University, Shanghai Engineering Research Center of Tooth Restoration and Regeneration, Shanghai 20072, China Received October 16, 2017; Accepted May 9, 2018; Epub August 15, 2018; Published August 30, 2018 Abstract: The aim of this study is to describe the anatomical characteristics of the ‘danger zone’ in human mandibu- lar first molars by measuring the thickness of the bending point (point α) and the thinnest point (point β) of each root in human mandibular first molars. Eighteen mandibular first molars were scanned by micro-computed tomography and reconstructed three-dimensionally by Mimics Research 17.0. To identify characteristics of the ‘danger zone’, the actual root curvature, the minimal dentin thickness of the bending slice and the thinnest slice were selected as parameters. Furthermore, to describe the relationship of point α and point β, the distance between them was also measured. The results showed that the actual canal curvatures in the mandibular first molars were 5-10 degrees larger than those measured in 2-D images including X-ray or CBCT. The thinnest thickness of roots in all canals was less than 1 mm except the two distal roots of three-rooted mandibular first molars. Moreover, as for some two-rooted mandibular first molars, their point α and point β were located in the same furcation surface of root, the result might be coincident in the mesiolingual side; whereas for three-rooted mandibular first molars, the distal two canals were found significantly thicker than the mesial two (p<0.05). -

Anatomical Analysis of the Resected Roots of Mandibular First Molars After Failed Non-Surgical Retreatment
Restor Dent Endod. 2018 May;43(2):e16 https://doi.org/10.5395/rde.2018.43.e16 pISSN 2234-7658·eISSN 2234-7666 Research Article Anatomical analysis of the resected roots of mandibular first molars after failed non-surgical retreatment Jiyoung Yoon ,1 Byeong-Hoon Cho ,2 Jihyun Bae ,1 Yonghoon Choi 1* 1Department of Conservative Dentistry, Section of Dentistry, Seoul National University Bundang Hospital, Seongnam, Korea 2Department of Conservative Dentistry, Seoul National University School of Dentistry and Dental Research Institute, Seoul, Korea Received: Nov 29, 2017 ABSTRACT Accepted: Jan 26, 2018 Objectives: Yoon J, Cho BH, Bae J, Choi Y Understanding the reason for an unsuccessful non-surgical endodontic treatment outcome, as well as the complex anatomy of the root canal system, is very *Correspondence to important. This study examined the cross-sectional root canal structure of mandibular first Yong-Hoon Choi, DDS, MSD, PhD molars confirmed to have failed non-surgical root canal treatment using digital images Associate Professor, Department of Conservative Dentistry, Section of Dentistry, obtained during intentional replantation surgery, as well as the causative factors of the failed Seoul National University Bundang Hospital, conventional endodontic treatments. 82 Gumi-ro 173-beon-gil, Bundang-gu, Materials and Methods: This study evaluated 115 mandibular first molars. Digital Seongnam 13620, Korea. photographic images of the resected surface were taken at the apical 3 mm level and E-mail: [email protected] examined. The discolored dentin area around the root canal was investigated by measuring Copyright © 2018. The Korean Academy of the total surface area, the treated areas as determined by the endodontic filling material, and Conservative Dentistry the discolored dentin area. -

Comparative Morphology of Incisor Enamel and Dentin in Humans and Fat Dormice (Glis Glis)
Coll. Antropol. 27 (2003) 1: 373–380 UDC 572.72:616.314.11 Original scientific paper Comparative Morphology of Incisor Enamel and Dentin in Humans and Fat Dormice (Glis glis) Dean Konjevi}1, Tomislav Keros2, Hrvoje Brki}3, Alen Slavica1, Zdravko Janicki1 and Josip Margaleti}4 1 Chair for Game Biology, Pathology and Breeding, Veterinary Faculty, University of Zagreb, Zagreb, Croatia 2 Croatian Veterinary Institute, Zagreb, Croatia 3 Department for Dental Anthropology, School of Dental Medicine, University of Zagreb, Zagreb, Croatia 4 Department of Forest Protection and Wildlife Management, Faculty of Forestry, University of Zagreb, Zagreb, Croatia ABSTRACT The structure of teeth in all living beings is genetically predetermined, although it can change under external physiological and pathological factors. The author’s hypoth- esis was to indicate evolutional shifts resulting from genetic, functional and other dif- ferences. A comparative study about certain characteristics of incisors in humans and myomorpha, the fat dormouse (Glis glis) being their representative as well, comprised measurements of enamel and dentin thickness in individual incisor segments, evalua- tion of external enamel index, and also assessment of histological structure of enamel and dentin. The study results involving dormice showed the enamel to be thicker in lower than in the upper teeth, quite contrary to enamel thickness in humans. In the up- per incisors in dormice the enamel is the thickest in the medial layer of the crown, and in the cervical portion of the crown in the lower incisors. The thickness of dentin in dor- mice is greater in the oral than in the vestibular side. These findings significantly differ from those reported in reference literature, but they are based on the function of teeth in dormice. -
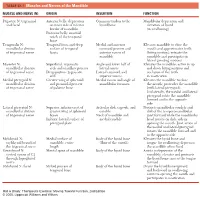
1 TABLE 23-1 Muscles and Nerves of the Mandible
0350 ch 23-Tab 10/12/04 12:19 PM Page 1 Chapter 23: The Temporomandibular Joint 1 TABLE 23-1 Muscles and Nerves of the Mandible MUSCLE AND NERVE (N) ORIGIN INSERTION FUNCTION Digastric N: trigeminal Anterior belly: depression Common tendon to the Mandibular depression and and facial on inner side of inferior hyoid bone elevation of hyoid border of mandible (in swallowing) Posterior belly: mastoid notch of the temporal bone Temporalis N: Temporal fossa and deep Medial and anterior Elevates mandible to close the mandibular division surface of temporal coronoid process and mouth and approximates teeth of trigeminal nerve fascia anterior ramus of (biting motion); retracts the mandible mandible and participates in lateral grinding motions Masseter N: Superficial: zygomatic Angle and lower half of Elevates the mandible; active in up mandibular division arch and maxillary process lateral ramus and down biting motions and of trigeminal nerve Deep portion: zygomatic Lateral coronoid and occlusion of the teeth arch superior ramus in mastication Medial pterygoid N: Greater wing of sphenoid Medial ramus and angle of Elevates the mandible to close mandibular division and pyramidal process mandibular foramen the mouth; protrudes the mandible of trigeminal nerve of palatine bone (with lateral pterygoid). Unilaterally, the medial and lateral pterygoid rotate the mandible forward and to the opposite side Lateral pterygoid N: Superior: inferior crest of Articular disk, capsule, and Protracts mandibular condyle and mandibular division greater wing of sphenoid condyle disk of the temporomandibular of trigeminal nerve bones Neck of mandible and joint forward while the mandibular Inferior: lateral surface of medial condyle head rotates on disk; aids in pterygoid plate opening the mouth. -

Bonding to Enamel and Dentin
Chapter 16 Bonding to Enamel and Dentin INTRODUCTION • Evolution of Dentin Bonding Agents ADHESIVE DENTISTRY • Nanofilled Bonding Agents • Indications for Use of Adhesives HYBRID LAYER AND HYBRIDIZATION • Advantages of Bonding Techniques • Hybridization (Given by Nakabayachi in 1982) • Mechanism of Adhesion • Zones of Hybrid Layer • Factors Affecting Adhesion SMEAR LAYER ENAMEL BONDING • Structure • Steps for Enamel Bonding • Formation of Smear Layer • Mechanism of Etching • Components of Smear Layer DENTIN BONDING • Role of Smear Layer • Conditioning of Dentin • Role of Smear Layer in Dentin Bonding • Priming of Dentin • Classification of Modern Adhesives • Moist vs Dry Dentin GLASS IONOMER BASED ADHESIVE SYSTEM DENTIN BONDING AGENT • Steps • Mechanism of Bonding FAILURE OF DENTIN BONDING INTRODUCTION fluid present in dentinal tubules makes the bonding difficult. The adhesion to dentin requires decalcification The traditional “drill and fill” approach is fading now of the dentinal surface to expose a layer of interlacing because of numerous advancements taking place in collagen fibrils and the entrances of the dentinal tubules. restorative dentistry. For a restorative material, adhesion When resins are used, they form an intermediate layer is the primary requirement so that restorative materials with exposed spongy collagen network which can be then can be bonded to enamel or dentin and without the need bonded to the retentive inner surface of the restoration of extensive tooth preparation. The initial advancement by means of a resin similar to that of enamel bonding. was made in 1956, by a pedodontist, Buonocore, who The past decade has seen increased use of bonding developed acid etching of the enamel. He showed that agents in concurrence with traditional dental materials. -

Illustrated Review of the Embryology and Development of the Facial
REVIEW ARTICLE Illustrated Review of the Embryology and Development of the Facial Region, Part 2: Late Development of the Fetal Face and Changes in the Face from the Newborn to Adulthood P.M. Som and T.P. Naidich ABSTRACT SUMMARY: The later embryogenesis of the fetal face and the alteration in the facial structure from birth to adulthood have been reviewed. Part 3 of the review will address the molecular mechanisms that are responsible for the changes described in parts 1 and 2. art 1 of this 3-part review primarily dealt with the early em- first make contact, each is completely covered by a homoge- Pbryologic development of the face and nasal cavity. Part 2 will neous epithelium. A special epithelium arises at the edge of discuss the later embryonic and fetal development of the face, and each palatal shelf, facilitating the eventual fusion of these changes in facial appearance from neonate to adulthood will be shelves. The epithelium on the nasal cavity surface of the palate reviewed. will differentiate into columnar ciliated epithelium. The epi- thelium on the oral cavity side of the palate will differentiate Formation of the Palate into stratified squamous epithelium. Between the sixth and 12th weeks, the palate is formed from 3 The 2 palatal shelves also fuse with the triangular primary pal- primordia: a midline median palatine process and paired lateral ate anteromedially to form a y-shaped fusion line. The point of palatine processes (Fig 1). In the beginning of the sixth week, fusion of the secondary palatal shelves with the primary palate is merging of the paired medial nasal processes forms the intermax- marked in the adult by the incisive foramen.