HCF Seminar 2017 Final Powerpoint
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Healthcare Transactions: Year in Review
HEALTHCARE TRANSACTIONS: YEAR IN REVIEW JANUARY 2019 bassberry.com OVERVIEW 2018 marked a year of continued robust healthcare merger and acquisition activity, with deal volume surpassing that of 2017. The dollar volume of healthcare deals also exceeded 2017, highlighted by CVS Health Corp. (NYSE: CVS) surviving regulatory scrutiny and finalizing its purchase of Aetna for $69 billion, and Cigna (NYSE: CI) closing its $67 billion merger with pharmacy benefit manager Express Scripts (Nasdaq: ESRX). Private equity firm KKR & Co. Inc. (NYSE: KKR) acquired Envision Healthcare Corporation (NYSE: EVHC) in a going private transaction for $9.9 billion; and in November, LifePoint Health, Inc. (Nasdaq: LPNT) merged with Apollo Global Management-owned RCCH HealthCare Partners for an estimated $5.6 billion. Not only do these deals show the energy of the industry, but they also represent a seismic shift in healthcare. Cross-sector transactions, such as the CVS-Aetna and Cigna-Express Scripts transactions, are becoming more frequent and disrupt traditional industry models. By seeking alliances across industry lines, healthcare companies aim both to reduce costs and improve care coordination as well as realign incentives to better meet consumer demands. As providers fight to stay profitable in the wake of value-based reimbursement, these cross-sector combinations may foreshadow the future of the healthcare industry. Many of these same drivers also have led to increasing private equity investment in the industry. The need for innovation and disruption via consolidation adds further appeal to investors. The injection of capital by private equity firms allows healthcare providers to cut costs and increase efficiencies, and the shift in reimbursement presents a unique opportunity for firms to help providers and systems reposition. -
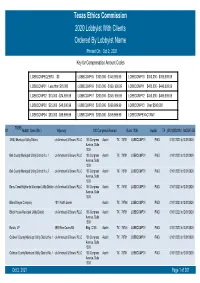
Texas Ethics Commission 2020 Lobbyist with Clients Ordered by Lobbyist Name Printed on Oct 2, 2021 Key for Compensation Amount Codes
Texas Ethics Commission 2020 Lobbyist With Clients Ordered By Lobbyist Name Printed On Oct 2, 2021 Key for Compensation Amount Codes LOBBCOMPEQZERO $0 LOBBCOMP05 $100,000 - $149,999.99 LOBBCOMP10 $350,000 - $399,999.99 LOBBCOMP01 Less than $10,000 LOBBCOMP06 $150,000 - $199, 999.99 LOBBCOMP11 $400,000 - $449,999.99 LOBBCOMP02 $10,000 - $24,999.99 LOBBCOMP07 $200,000 - $249, 999.99 LOBBCOMP12 $450,000 - $499,999.99 LOBBCOMP03 $25,000 - $49,000.99 LOBBCOMP08 $250,000 - $299,999.99 LOBBCOMP13 Over $500,000 LOBBCOMP04 $50,000 - $99,999.99 LOBBCOMP09 $300,000 - $349,999.99 LOBBCOMPEXACTAMT 70358 #1 Abbott, Sean (Mr.) Attorney 100 Congress Avenue Suite 1300 Austin TX (512)4352334 MODIFIED 3 B&J Municipal Utility District c/o Armbrust & Brown, PLLC 100 Congress Austin TX 78701 LOBBCOMP01 PAID 01/01/2020 to12/31/2020 Avenue, Suite 1300 Bell County Municipal Utility District No. 1 c/o Armbrust & Brown, PLLC 100 Congress Austin TX 78701 LOBBCOMP01 PAID 01/01/2020 to12/31/2020 Avenue, Suite 1300 Bell County Municipal Utility District No. 2 c/o Armbrust & Brown, PLLC 100 Congress Austin TX 78701 LOBBCOMP01 PAID 01/01/2020 to12/31/2020 Avenue, Suite 1300 Berry Creek Highlands Municipal Utility District c/o Armbrust & Brown, PLLC 100 Congress Austin TX 78701 LOBBCOMP01 PAID 01/01/2020 to12/31/2020 Avenue, Suite 1300 Blake Magee Company 1011 North Lamar Austin TX 78704 LOBBCOMP01 PAID 01/01/2020 to12/31/2020 Block House Municipal Utility District c/o Armbrust & Brown, PLLC 100 Congress Austin TX 78701 LOBBCOMP01 PAID 01/01/2020 to12/31/2020 Avenue, Suite 1300 Bonzo, LP 3939 Bee Caves Rd. -

Private Equity Buyouts in Healthcare: Who Wins, Who Loses? Eileen Appelbaum and Rosemary Batt Working Paper No
Private Equity Buyouts in Healthcare: Who Wins, Who Loses? Eileen Appelbaum* and Rosemary Batt† Working Paper No. 118 March 15, 2020 ABSTRACT Private equity firms have become major players in the healthcare industry. How has this happened and what are the results? What is private equity’s ‘value proposition’ to the industry and to the American people -- at a time when healthcare is under constant pressure to cut costs and prices? How can PE firms use their classic leveraged buyout model to ‘save healthcare’ while delivering ‘outsized returns’ to investors? In this paper, we bring together a wide range of sources and empirical evidence to answer these questions. Given the complexity of the sector, we focus on four segments where private equity firms have been particularly active: hospitals, outpatient care (urgent care and ambulatory surgery centers), physician staffing and emergency * Co-Director and Senior Economist, Center for Economic and Policy Research. [email protected] † Alice H. Cook Professor of Women and Work, HR Studies and Intl. & Comparative Labor ILR School, Cornell University. [email protected]. We thank Andrea Beaty, Aimee La France, and Kellie Franzblau for able research assistance. room services (surprise medical billing), and revenue cycle management (medical debt collecting). In each of these segments, private equity has taken the lead in consolidating small providers, loading them with debt, and rolling them up into large powerhouses with substantial market power before exiting with handsome returns. https://doi.org/10.36687/inetwp118 JEL Codes: I11 G23 G34 Keywords: Private Equity, Leveraged Buyouts, health care industry, financial engineering, surprise medical billing revenue cycle management, urgent care, ambulatory care. -

Healthcare Real Estate
VALUE FOCUS REAL ESTATE Sector Focus: Healthcare Real Estate Second Quarter 2017 Healthcare Facilities 1 Macro Indicators 3 Industry Performance and M&A Activity 6 Publicly Traded Companies Hospitality 9 Residential 11 Healthcare 12 Commercial Real Estate 16 About Mercer Capital 22 www.mercercapital.com Mercer Capital’s Value Focus: Real Estate Industry Second Quarter 2017 Healthcare Facilities As of the most recent data in 2015, healthcare spending in the United States was $3.2 trillion. Average annual growth in national healthcare expenditures from 2015 to 2025 is expected to be approximately 5.6%. As a share of gross domestic product, national healthcare expenditure is expected to increase from 17.8% in 2015 to 19.9% by 2025. Demand for medical office buildings (MOBs) is driven primarily by demographic characteristics, rather than economic trends. This has contributed to more stable performance for the medical office building segment during a period when office space in general has underperformed. Consolidation continued in the industry during 2016, spurred by efforts to cut costs and offset lower insurance reimbursement rates. This trend has encouraged demand for larger office space and weakened the market for smaller, individual healthcare facilities, although ambulatory centers associated with larger hospitals are an exception. Merger and acquisition activity is expected to pick up in 2017, following an 8.0% increase in transactions in the first quarter. Of 27 transactions during the first quarter, three included organizations with revenues of $1 billion or greater. Average rents in the medical office space were $24.00 per square foot in 2016, which represents an 8.0% increase over the prior year and 8.3% above the $22.16 low observed in early 2013. -

051820 Tractor Supply
OFFERING MEMORANDUM TRACTOR SUPPLY – HOHENWALD, TN 608 E Main Street • Hohenwald, TN 38462 Representative Photo NON- ENDORSEMENT AND DISCLAIMER NOTICE Confidentiality and Disclaimer The information contained in the following Marketing Brochure is proprietary and strictly confidential. It is intended to be reviewed only by the party receiving it from Marcus & Millichap and should not be made available to any other person or entity without the written consent of Marcus & Millichap. This Marketing Brochure has been prepared to provide summary, unverified information to prospective purchasers, and to establish only a preliminary level of interest in the subject property. The information contained herein is not a substitute for a thorough due diligence investigation. Marcus & Millichap has not made any investigation, and makes no warranty or representation, with respect to the income or expenses for the subject property, the future projected financial performance of the property, the size and square footage of the property and improvements, the presence or absence of contaminating substances, PCB's or asbestos, the compliance with State and Federal regulations, the physical condition of the improvements thereon, or the financial condition or business prospects of any tenant, or any tenant's plans or intentions to continue its occupancy of the subject property. The information contained in this Marketing Brochure has been obtained from sources we believe to be reliable; however, Marcus & Millichap has not verified, and will not verify, any of the information contained herein, nor has Marcus & Millichap conducted any investigation regarding these matters and makes no warranty or representation whatsoever regarding the accuracy or completeness of the information provided. -

Nashville Healthcare Fraud Conference
Bass, Berry & Sims The Pinnacle at Symphony Place NASHVILLE 150 Third Avenue South, Suite 2800 Nashville, Tennessee 37201 HEALTHCARE FRAUD CONFERENCE THURSDAY DECEMBER 7, 2017 SCHEDULE 7:30 – 8:30 Registration and Breakfast 8:30 – 8:40 Welcome 8:40 – 9:20 Healthcare Fraud Year in Review: Where Do We Go From Here? Lindsey Fetzer, Associate, Bass, Berry & Sims PLC Brian Roark, Member, Bass, Berry & Sims PLC 9:25 – 9:55 Effectively Handling Employment Decisions Involving Whistleblowers (Ethics CLE) Justin Page, Deputy General Counsel, Ambulatory Services, Envision Healthcare Corporation Taylor Chenery, Member, Bass, Berry & Sims PLC 9:55 – 10:10 Break 10:10 – 10:45 What Every Healthcare Compliance Officer Wishes the Legal Department Knew LeToia Crozier, Chief Compliance Officer, Change Healthcare Amy Garner, Vice President and Chief Compliance Officer, West Tennessee Healthcare Steve Hinkle, Vice President and Chief Compliance Officer, Ardent Health Services Moderated by: Anna Grizzle, Member, Bass, Berry & Sims PLC 10:50 – 11:25 Mine Your Own Data: The Role of Data in Dealing with Healthcare Fraud Issues Greg Russo, Managing Director, Berkeley Research Group Jeff Gibson, Associate, Bass, Berry & Sims PLC 11:25 – 12:00 Revisiting Parallel Proceedings in Healthcare Fraud Cases Stuart Canale, Assistant U.S. Attorney, U.S. Attorney’s Office for the Western District of Tennessee Mary Jane Stewart, First Assistant U.S. Attorney, U.S. Attorney’s Office for the Middle District of Tennessee Lisa Rivera, Member, Bass, Berry & Sims PLC 12:00 – 12:30 Lunch Buffet 12:30 – 1:30 Keynote Presentation: A View from the U.S. Attorneys Hon. Donald Q. -

Board of Directors' Pay up from Last Year
Published By 200 Business Park Drive Armonk, NY 10504 Phone: 914.730.7300 Fax: 914.730.7303 www.total-comp.com 2017 / 2018 BOARD OF DIRECTORS COMPENSATION REPORT January 2018 All rights reserved. © 2018 Total Compensation Solutions, LLC. Printed in the United States of America. This publication of the 2017/2018 Board of Directors Compensation Report may not be reproduced, stored in a retrieval system, or transmitted in whole or in part, in any form or by any means (electronic, mechanical, photocopying, recording or otherwise) without the prior written permission of Total Compensation Solutions, LLC. 200 Business Park Drive, Armonk, NY 10504. The information provided in this report is confidential and for the use of the subscribing organization only. By accepting this material, you agree that it will not be reproduced, copied, transmitted or disclosed to organizations or persons outside of your organization. Table of Contents Section Page I. Executive Summary Introduction ------------------------------------------------------------------- 2 Company Demographics -------------------------------------------------- 5 Findings and Observations ----------------------------------------------- 8 II. Data Analysis Board Structure -------------------------------------------------------------- 13 Committee Structure ------------------------------------------------------- 16 Board Compensation ------------------------------------------------------ 19 III. Board Compensation All Companies -------------------------------------------------------------- -

Digit Al Annu Al Report
DIGITAL ANNUAL REPORT Explore more of the story with FROM THE CHAIRMAN The 2019-2020 fiscal year ended in the midst of a global pandemic, significant social unrest, and the worst economy since the Great Depression. Great challenges call for great leadership—and in health care—we have all seen the nation’s health care industry step-up in heroic ways to fight COVID-19 and focus on the health and well-being of our communities. The Nashville Health Care Council, its leadership, and its member companies demonstrated such leadership in numerous collaborative efforts as you will read about in this annual report. Alongside the backdrop of the coronavirus pandemic during 2020, the full-year operational performance of the Nashville Health Care Council was strong, setting a solid foundation for future growth and national leadership for the health care industry. In the 2019-2020 fiscal year, 45 companies joined the Nashville Health Care Council as new members, expanding the scope and diversity of companies represented in its roster of over 300 members. Member companies remained highly engaged throughout the year. Over 100 notable speakers participated in a variety of targeting programming throughout the year. The prestigious Nashville Health Care Council Fellows program was held with 30 highly esteemed, executive health care leaders from across the industry. A delegation of 50 members Robert A. Frist, Jr. (Chairman) traveled to Boston, Massachusetts through the Leadership Health Care program where innovative Co-founder, CEO developments in health care delivery were explored, discussed, and shared with other industry and Chairman leaders in the area. -
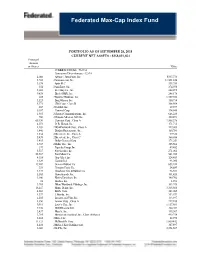
Federated Max-Cap Index Fund
Federated Max-Cap Index Fund PORTFOLIO AS OF SEPTEMBER 28, 2018 CURRENT NET ASSETS - $358,021,654 Principal Amount or Shares Value COMMON STOCKS - 96.6%1 Consumer Discretionary - 12.6% 2,066 Advance Auto Parts, Inc. $347,770 5,742 2 Amazon.com, Inc. 11,501,226 3,978 Aptiv PLC 333,754 354 2 AutoZone, Inc. 274,598 5,601 Best Buy Co., Inc. 444,495 9,638 Block (H&R), Inc. 248,178 685 2 Booking Holdings, Inc. 1,359,040 3,523 BorgWarner, Inc. 150,714 5,371 CBS Corp., Class B 308,564 267 2 CarMax, Inc. 19,937 6,187 Carnival Corp. 394,545 1,983 2 Charter Communications, Inc. 646,220 706 2 Chipotle Mexican Grill, Inc. 320,891 65,190 Comcast Corp., Class A 2,308,378 4,071 D. R. Horton, Inc. 171,715 4,169 2 DISH Network Corp., Class A 149,083 1,446 Darden Restaurants, Inc. 160,781 1,854 2 Discovery, Inc., Class A 59,328 5,439 2 Discovery, Inc., Class C 160,886 3,415 Dollar General Corp. 373,259 3,747 2 Dollar Tree, Inc. 305,568 379 Expedia Group, Inc. 49,452 5,317 Foot Locker, Inc. 271,061 58,567 Ford Motor Co. 541,745 4,314 Gap (The), Inc. 124,459 1,329 Garmin Ltd. 93,096 19,407 General Motors Co. 653,434 351 Genuine Parts Co. 34,889 4,127 Goodyear Tire & Rubber Co. 96,531 5,503 Hanesbrands, Inc. 101,420 3,106 Harley Davidson, Inc. 140,702 18 Hasbro, Inc. -

2019-2020 Annual Report
OUR PURPOSE: Nashville is a thriving city filled with risk-takers. Positive energy and camaraderie fuel the spirit of the region. Every day people find new ways to take advantage of opportunities offered, to make something from nothing, to make their mark, to realize their dreams. At the center of this spirit is the Nashville Area Chamber of Commerce. 2 2O19-2O2O THANK YOU TO OUR SPONSORS PIVOTAL PARTNERS FRIENDS OF THE CHAMBER PRESENTING SPONSORS ASSOCIATE SPONSORS Baker Donelson JPMorgan Chase & Co. Crown Castle BKD, LLP Lipscomb University Pfeffer Sertoma Club of Nashville CoreCivic Graduate School of Business Vanderbilt Executive Education DevDigital Piedmont Natural Gas First Horizon Bank PNC Bank Frost Brown Todd LLC SUPPORTING SPONSORS Bank of America KraftCPAs, PLLC Barge Design Solutions Lipscomb Spark Belmont University Center Metro Nashville Airport for International Business Authority FirstBank Mitsui & Co. (U.S.A.), Inc. Gallagher Osborn Barr | Paramore Google Fiber Ragan-Smith Associates, Inc. Greater Nashville Realtors® Southwest Airlines TECHNOLOGY PARTNER OFFICE SOLUTIONS PARTNER Atiba Novatech MEDIA PARTNERS FW Publishing Lightning 100 Tennessean | LOCALiQ SUPPORTING SPONSORS Ajax Turner Comcast Business Altria Price CPAs Bank of Tennessee Royal Cup Coffee Batch Nashville Sound Royalties Big Machine Vodka U.S. Army Recruiting Battalion Contagious Cocktails 3 NASHVILLE AREA CHAMBER OF COMMERCE 2O2O - 2O21 BOARD OF DIRECTORS CHAIR WANDA LYLE Managing Director, General Manager, UBS Business Solutions US LLC | Nashville IMMEDIATE PAST CHAIR ROBERT A. MCCABE, JR. Chairman, Pinnacle Financial Partners VICE CHAIR TODD ROLAPP Managing Partner, Bass Berry & Sims SECRETARY CAROL YOCHEM President Middle Tennessee Region, First Horizon PRESIDENT RALPH SCHULZ President & CEO, Nashville Area Chamber of Commerce GENERAL COUNSEL CAROLYN W. -

Global Healthcare Private Equity and Corporate M&A Report 2019
Global Healthcare Private Equity and Corporate M&A Report 2019 About Bain & Company’s Private Equity business Bain & Company is the leading consulting partner to the private equity (PE) industry and its stake- holders. PE consulting at Bain has grown eightfold over the past 15 years and now represents about one-quarter of the firm’s global business. We maintain a global network of more than 1,000 experi- enced professionals serving PE clients. Our practice is more than triple the size of the next largest consulting company serving PE firms. Bain’s work with PE firms spans fund types, including buyout, infrastructure, real estate and debt. We also work with hedge funds, as well as many of the most prominent institutional investors, in- cluding sovereign wealth funds, pension funds, endowments and family investment offices. We sup- port our clients across a broad range of objectives: Deal generation. We help develop differentiated investment theses and enhance deal flow by profiling industries, screening companies and devising a plan to approach targets. Due diligence. We help support better deal decisions by performing integrated due diligence to assess the market dynamics, a target’s competitive position and margin expansion opportunities, and by providing a post-acquisition agenda. Immediate post-acquisition. We support the pursuit of rapid returns by developing a strategic value- creation plan for the acquired company, leading workshops that align management with strategic priorities and directing focused initiatives or wholesale transformations. Ongoing value addition. We help increase company value by supporting revenue enhancement and cost reduction and by refreshing strategy. Exit. -
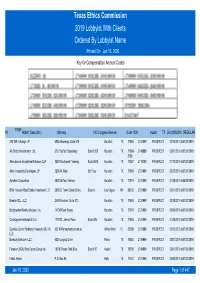
Texas Ethics Commission 2019 Lobbyist with Clients Ordered by Lobbyist Name Printed on Jan 10, 2020 Key for Compensation Amount Codes
Texas Ethics Commission 2019 Lobbyist With Clients Ordered By Lobbyist Name Printed On Jan 10, 2020 Key for Compensation Amount Codes 70358 #1 Abbott, Sean (Mr.) Attorney 100 Congress Avenue Suite 1300 Austin TX (512)4352334 REGULAR 290 WR Holdings LP 5005 Riverway, Suite 500 Houston TX 77056 LT24999 PROSPECT 02/12/2019 to07/31/2019 AA Sharp Investments Ltd. 3737 Buffalo Speedway Suite 1500 Houston TX 77098- LT49999 PROSPECT 03/01/2019 to07/31/2019 3738 Allen Boone Humphries Robinson LLP 3200 Southwest Freeway Suite 2600 Houston TX 77027 LT10000 PROSPECT 01/10/2019 to07/31/2019 Allen Hospitality Developers LP 2506 W. Main 5th Floor Houston TX 77098 LT24999 PROSPECT 03/20/2019 to07/31/2019 Ayrshire Corporation 2028 Buffalo Terrace Houston TX 77019 LT24999 PROSPECT 01/28/2019 to05/09/2019 BAM Houston Real Estate Investment L.P. 3605 S. Town Center Drive Suite A Las Vegas NV 89135 LT24999 PROSPECT 03/11/2019 to07/31/2019 Brende SCL LLC 2450 Fondren, Suite 210 Houston TX 77063 LT24999 PROSPECT 02/18/2019 to07/31/2019 Bridgewater Realty Advisors Inc. 14738 River Forest Houston TX 77079 LT24999 PROSPECT 05/09/2019 to07/31/2019 Cunningham Interests II Ltd 1770 St. James Place Suite 205 Houston TX 77056 LT24999 PROSPECT 01/28/2019 to07/31/2019 Cypress Grand Parkway (Houston) ASLI IX 923 N Pennsylvania Avenue Winter Park FL 32789 LT24999 PROSPECT 02/12/2019 to07/31/2019 LLC Denbury Onshore LLC 5320 Legacy Drive Plano TX 75024 LT49999 PROSPECT 03/01/2019 to07/31/2019 Forestar (USA) Real Estate Group Inc.