Tax O Xonomic of the Gen C and Phy Nus Pota Ylogenet Amon (Cr F Tic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
The Freshwater Snails (Gastropoda) of Iran, with Descriptions of Two New Genera and Eight New Species
A peer-reviewed open-access journal ZooKeys 219: The11–61 freshwater (2012) snails (Gastropoda) of Iran, with descriptions of two new genera... 11 doi: 10.3897/zookeys.219.3406 RESEARCH articLE www.zookeys.org Launched to accelerate biodiversity research The freshwater snails (Gastropoda) of Iran, with descriptions of two new genera and eight new species Peter Glöer1,†, Vladimir Pešić2,‡ 1 Biodiversity Research Laboratory, Schulstraße 3, D-25491 Hetlingen, Germany 2 Department of Biology, Faculty of Sciences, University of Montenegro, Cetinjski put b.b., 81000 Podgorica, Montenegro † urn:lsid:zoobank.org:author:8CB6BA7C-D04E-4586-BA1D-72FAFF54C4C9 ‡ urn:lsid:zoobank.org:author:719843C2-B25C-4F8B-A063-946F53CB6327 Corresponding author: Vladimir Pešić ([email protected]) Academic editor: Eike Neubert | Received 18 May 2012 | Accepted 24 August 2012 | Published 4 September 2012 urn:lsid:zoobank.org:pub:35A0EBEF-8157-40B5-BE49-9DBD7B273918 Citation: Glöer P, Pešić V (2012) The freshwater snails (Gastropoda) of Iran, with descriptions of two new genera and eight new species. ZooKeys 219: 11–61. doi: 10.3897/zookeys.219.3406 Abstract Using published records and original data from recent field work and revision of Iranian material of cer- tain species deposited in the collections of the Natural History Museum Basel, the Zoological Museum Berlin, and Natural History Museum Vienna, a checklist of the freshwater gastropod fauna of Iran was compiled. This checklist contains 73 species from 34 genera and 14 families of freshwater snails; 27 of these species (37%) are endemic to Iran. Two new genera, Kaskakia and Sarkhia, and eight species, i.e., Bithynia forcarti, B. starmuehlneri, B. -

Crustacea-Arthropoda) Fauna of Sinop and Samsun and Their Ecology
J. Black Sea/Mediterranean Environment Vol. 15: 47- 60 (2009) Freshwater and brackish water Malacostraca (Crustacea-Arthropoda) fauna of Sinop and Samsun and their ecology Sinop ve Samsun illeri tatlısu ve acısu Malacostraca (Crustacea-Arthropoda) faunası ve ekolojileri Mehmet Akbulut1*, M. Ruşen Ustaoğlu2, Ekrem Şanver Çelik1 1 Çanakkale Onsekiz Mart University, Fisheries Faculty, Çanakkale-Turkey 2 Ege University, Fisheries Faculty, Izmir-Turkey Abstract Malacostraca fauna collected from freshwater and brackishwater in Sinop and Samsun were studied from 181 stations between February 1999 and September 2000. 19 species and 4 subspecies belonging to 15 genuses were found in 134 stations. In total, 23 taxon were found: 11 Amphipoda, 6 Decapoda, 4 Isopoda, and 2 Mysidacea. Limnomysis benedeni is the first time in Turkish Mysidacea fauna. In this work at the first time recorded group are Gammarus pulex pulex, Gammarus aequicauda, Gammarus uludagi, Gammarus komareki, Gammarus longipedis, Gammarus balcanicus, Echinogammarus ischnus, Orchestia stephenseni Paramysis kosswigi, Idotea baltica basteri, Idotea hectica, Sphaeroma serratum, Palaemon adspersus, Crangon crangon, Potamon ibericum tauricum and Carcinus aestuarii in the studied area. Potamon ibericum tauricum is the most encountered and widespread species. Key words: Freshwater, brackish water, Malacostraca, Sinop, Samsun, Turkey Introduction The Malacostraca is the largest subgroup of crustaceans and includes the decapods such as crabs, mole crabs, lobsters, true shrimps and the stomatopods or mantis shrimps. There are more than 22,000 taxa in this group representing two third of all crustacean species and contains all the larger forms. *Corresponding author: [email protected] 47 Malacostracans play an important role in aquatic ecosystems and therefore their conservation is important. -

Circadian Clocks in Crustaceans: Identified Neuronal and Cellular Systems
Circadian clocks in crustaceans: identified neuronal and cellular systems Johannes Strauss, Heinrich Dircksen Department of Zoology, Stockholm University, Svante Arrhenius vag 18A, S-10691 Stockholm, Sweden TABLE OF CONTENTS 1. Abstract 2. Introduction: crustacean circadian biology 2.1. Rhythms and circadian phenomena 2.2. Chronobiological systems in Crustacea 2.3. Pacemakers in crustacean circadian systems 3. The cellular basis of crustacean circadian rhythms 3.1. The retina of the eye 3.1.1. Eye pigment migration and its adaptive role 3.1.2. Receptor potential changes of retinular cells in the electroretinogram (ERG) 3.2. Eyestalk systems and mediators of circadian rhythmicity 3.2.1. Red pigment concentrating hormone (RPCH) 3.2.2. Crustacean hyperglycaemic hormone (CHH) 3.2.3. Pigment-dispersing hormone (PDH) 3.2.4. Serotonin 3.2.5. Melatonin 3.2.6. Further factors with possible effects on circadian rhythmicity 3.3. The caudal photoreceptor of the crayfish terminal abdominal ganglion (CPR) 3.4. Extraretinal brain photoreceptors 3.5. Integration of distributed circadian clock systems and rhythms 4. Comparative aspects of crustacean clocks 4.1. Evolution of circadian pacemakers in arthropods 4.2. Putative clock neurons conserved in crustaceans and insects 4.3. Clock genes in crustaceans 4.3.1. Current knowledge about insect clock genes 4.3.2. Crustacean clock-gene 4.3.3. Crustacean period-gene 4.3.4. Crustacean cryptochrome-gene 5. Perspective 6. Acknowledgements 7. References 1. ABSTRACT Circadian rhythms are known for locomotory and reproductive behaviours, and the functioning of sensory organs, nervous structures, metabolism and developmental processes. The mechanisms and cellular bases of control are mainly inferred from circadian phenomenologies, ablation experiments and pharmacological approaches. -

A New Species of Riverine Crab of the Genus Sundathelphusa Bott, 1969 (Crustacea: Brachyura: Gecarcinucidae) from Northeastern Luzon, Philippines
Philippine Journal of Science 139 (1): 61-70, June 2010 ISSN 0031 - 7683 A New Species of Riverine Crab of the Genus Sundathelphusa Bott, 1969 (Crustacea: Brachyura: Gecarcinucidae) from Northeastern Luzon, Philippines Jose Christopher E. Mendoza1,* and Tohru Naruse2 1Department of Biological Sciences, National University of Singapore, 14 Science Drive 4, 117543 Singapore 2Raffles Museum of Biodiversity Research, Department of Biological Sciences, National University of Singapore, 14 Science Drive 4, 117543 Singapore Present Address: Transdisciplinary Research Organization for Subtropical and Island Studies, University of the Ryukyus, 870 Uehara, Taketomi, Okinawa 907-1541, Japan A new species of riverine freshwater crab of the genus Sundathelphusa Bott, 1969, is described from Cagayan Province in northeastern Luzon Island, Philippines. Sundathelphusa cagayana, a new species, is most similar morphologically to other Philippine species with a subquadrate carapace such as S. antipoloensis (Rathbun, 1904), S. grapsoides (H. Milne Edwards, 1853) and S. wolterecki (Balss, 1937) but differs from each by characters of the carapace, epibranchial teeth, ambulatory legs, male abdomen, and gonopods. This discovery brings to 22 the total number of species of Sundathelphusa found in the Philippines. A list of the Sundathelphusa species presently known from the Philippines and their known localities is also provided. Key Words: biodiversity, Cagayan, freshwater crab, new species, Pinacanauan River, taxonomy, Philippines INTRODUCTION either brackish water or sea water. All freshwater crabs undergo direct development (whereby all larval stages are The Philippines has a wide diversity of true freshwater crabs lacking), where the ovigerous female broods a few large, belonging to the families Potamidae and Gecarcinucidae lecithotrophic eggs that hatch into tiny hatchling crabs (Takeda 1983; Ng 1991; Ng & Takeda 1992, 1993a, b; Ng (Ng 1988). -
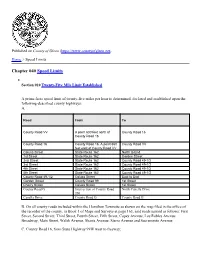
Speed Limits
Published on County of Glenn (https://www.countyofglenn.net) Home > Speed Limits Chapter 040 Speed Limits Section 010 Twenty-Five Mile Limit Established A prima facie speed limit of twenty-five miles per hour is determined, declared and established upon the following-described county highways: A. Road From To County Road VV A point 600 feet north of County Road 16 County Road 16 County Road 16 County Road 16, A point 660 County Road VV feet east of County Road VV Colusa Street State Route 162 North to End 1st Street State Route 162 Garden Street 2nd Street State Route 162 County Road 49-1/2 3rd Street State Route 162 County Road 49-1/2 4th Street State Route 162 County Road 49-1/2 5th Street State Route 162 County Road 49-1/2 County Road 49-1/2 Colusa Street East to End Garden Street County Road 99 1st Street Cherry Street Colusa Street 1st Street County Road G Intersection of County Road North Canella Drive 200 Canella Drive County Road G County Road G B. On all county roads included within the Hamilton Townsite as shown on the map filed in the office of the recorder of the county, in Book 1 of Maps and Surveys at page 163, said roads named as follows: First Street, Second Street, Third Street, Fourth Street, Fifth Street, Capay Avenue, Los Robles Avenue, Broadway, Main Street, Walsh Avenue, Shasta Avenue, Sierra Avenue and Sacramento Avenue; C. County Road 16, from State Highway 99W west to freeway; D. County Road 306, from the intersection of County Road 306 and County Road 309, southerly 2,640 ft; E. -

Who Is Who 1997
2nd Volume Convention on Climate Change Who is Who in the UNFCCC Process 1996 - 1997 FCCC Directory of Participants at Meetings of the Convention Bodies in the period July 1996 to December 1997 UN (COP2 - COP3) Contents Introduction page 3 Representatives of Countries page 5 Representatives of Observer Organizations page 259 Appendix I - Intergovernmental organizations accredited by the Conference of the Parties up to its third session page 482 Appendix II - Non-governmental organizations accredited by the Conference of the Parties up to its third session page 483 Appendix III - Alphabetical index of entries page 486 Appendix IV - Information update form page 523 1 2 Introduction This is the second volume of the Who’s Who in the UNFCCC Process. As indicated by its subtitle, this CC:INFO product is a directory of delegates and observers having attended the second or third sessions of the Conference of the Parties of the United Nations Framework Convention on Climate Change, or any of its subsidiary body meetings in between (COP2-COP3). This Who is Who was developed to provide those involved in the Climate Change process with a single, easy-to-use document, enabling them to renew or establish contact with each other. The Who is Who provides the title and contact information (e.g., institutional and e-mail addresses, direct telephone and fax numbers, etc…) for each individual, as provided to the secretariat during conference registration. Some of this information is now no longer valid, due to, e.g., new professional reassignments, including in some cases to the Climate Change Secretariat. -

Paratelphusa 13
--L - - • > 9^-: [h ' H '\:>t y^^ X-T-J ^ J^"i >w^%^' m -_'''_ .-r-r;. ,:-:?>?^ '* 1.1 :!;. ^ -A:?M^7A\ I 1- ^ I -I -• — V- --J K. 1 .li. T J-B ... __ ,.^-^,-^^ " • tail i:-' r=^ -/T 7>: ^ - •-;i"\ 4' f t-*i.?-i^H^ -• ' . 'V..- '- ^ '—I r^. ' ir < - '• •\ •• y. - ) .-• J - I ^ - ' '-•*. f ^ '•-•/ •-' '• -r —'•* - 1 - -• • ^ X ' I ^ ^' -' —i,.y J. -M-: '*;:: • -^ _1 *> •-. ::;. - ^ ••'.••- " - - - J-r-.,K • > A- ."• C •- :\ . - i- ' >* /. ,,/. -; ^YV ^^'' •"••• ^M ./" ^' ^ -:? jfj •^ ^ J ' b -IT .\ ^S ' V . 1 -I* ^. i'-4 J ClTAEOGf ••-'.•. r^- \x. J - ^ J h ,--^ d( • ••'•*• - -5 - ^ • •- - ' - -' I- 'W^c •, ' '' •••••• -1 Hi"'.''- -'<• u I - '* . ' ! I • -• ^. > y A- K L T ^ * ':\ ^' -^ -'^' % • > fr- ^ ' '' • J "^ vf, - ^ - - -1* >- — h .,>y OF THE r •' ^ v ,1 r.- / •/ . -v.-*. \ _ •-I '•r v>^,- " - ••• i-..,-v. "^ •• rs F- . v ^T^' f » **r • •>- >• » .,*. vV • J -' *• .V ^ _^ "J J V . L| ..**lr- n- -• -^ I ^ y- -•^ .t ^^ -'\ ,- s' m .>•-. *- ; '. b 'T^n -" •- % - ': I -' •» ; --< • \^ r% •-< '^ ' • - f n- -"l.ir'^ ' L "ij- .r '• ^1.. ^*r ^ --• --. 1 . -J -• -, •» --•• & .w - • : ^..^^'i >^ >s. (•.i.^ •: .T'^. -y 7 -•t -"•*> •^ .-.- : ' . '*- ii_ f r -^ "•-. < - > ' :•- • •-- . --# 'I f * •: J \ ^ k. ^1 -^•^ T y ef'TKE- - ' X H- *ff'' ^ V . *. •r' H r * Hi < 'T< »< -; _' _- .../•^ -• > \ .*^A » ' .' '• - • k - I- ^ . •>^>- --< */ A - ' - •.. :^ \-. 7* f • i "^ .- ^ -• -'/ .r' V •,! IT' « •«• ; - ^.' •. •' ** t. \ •v •V, /. ^' •^ ^.^ A. V, I 1 * -/ > ' I - * V v' *" t i*. Jl ' ^ V (« 1- I ^r \ ,\^ J. - , _ r >-• -\ ,) *- "- " 1^ •tjx N, /- r . •* r '".. '>.*>- >.^' .m- i •- -i. i_ ' --< -- -w '<^ 1^ - ^' I, f '^ r- - •»> •'. - ,<"' ^ y. ififVi' -I -- '. J OF THE . V, T\-: .--^ ' ' ' ^ r i*^ ' ^ \ J-l.'- \J- r - 't - 1 , 'I •^ ^^- II • ll -:*'^ X - Jt. •'J>..-- N-",; 31 -. 1 •.'! . -

Puerto Rico Comprehensive Wildlife Conservation Strategy 2005
Comprehensive Wildlife Conservation Strategy Puerto Rico PUERTO RICO COMPREHENSIVE WILDLIFE CONSERVATION STRATEGY 2005 Miguel A. García José A. Cruz-Burgos Eduardo Ventosa-Febles Ricardo López-Ortiz ii Comprehensive Wildlife Conservation Strategy Puerto Rico ACKNOWLEDGMENTS Financial support for the completion of this initiative was provided to the Puerto Rico Department of Natural and Environmental Resources (DNER) by U.S. Fish and Wildlife Service (USFWS) Federal Assistance Office. Special thanks to Mr. Michael L. Piccirilli, Ms. Nicole Jiménez-Cooper, Ms. Emily Jo Williams, and Ms. Christine Willis from the USFWS, Region 4, for their support through the preparation of this document. Thanks to the colleagues that participated in the Comprehensive Wildlife Conservation Strategy (CWCS) Steering Committee: Mr. Ramón F. Martínez, Mr. José Berríos, Mrs. Aida Rosario, Mr. José Chabert, and Dr. Craig Lilyestrom for their collaboration in different aspects of this strategy. Other colleagues from DNER also contributed significantly to complete this document within the limited time schedule: Ms. María Camacho, Mr. Ramón L. Rivera, Ms. Griselle Rodríguez Ferrer, Mr. Alberto Puente, Mr. José Sustache, Ms. María M. Santiago, Mrs. María de Lourdes Olmeda, Mr. Gustavo Olivieri, Mrs. Vanessa Gautier, Ms. Hana Y. López-Torres, Mrs. Carmen Cardona, and Mr. Iván Llerandi-Román. Also, special thanks to Mr. Juan Luis Martínez from the University of Puerto Rico, for designing the cover of this document. A number of collaborators participated in earlier revisions of this CWCS: Mr. Fernando Nuñez-García, Mr. José Berríos, Dr. Craig Lilyestrom, Mr. Miguel Figuerola and Mr. Leopoldo Miranda. A special recognition goes to the authors and collaborators of the supporting documents, particularly, Regulation No. -

Bidding, Contract Documents and Specifications For
Bidding, Contract Documents and Specifications for 2018 HMA Overlay Projects: Bid Package #2018-HMA-01 Bid Package #2018-SUB-01 NOBLE COUNTY, INDIANA NOBLE COUNTY BOARD OF COMMISSIONERS Gary Leatherman, President Dave Dolezal, Vice President Anita Hess, Member Prepared by: Zachary S. Smith, P.E. Date: May 8, 2017 1118 E. Main St. Albion, IN 46701 260-636-2124 [email protected] NOTICE TO BIDDERS Notice is hereby given by the Noble County Board of Commissioners that they will receive sealed bids for the following; Bid Package # 2018-HMA-01 Bid Package # 2018-SUB-01 Sealed bids are to be received in the Auditor’s Office by 2:30 pm on June 22, 2018. Mail or deliver to the Noble County Auditor, 101 N. Orange St., Albion, IN 46701. Bids will be opened during the Commissioner's meeting in the Commissioner’s Room at the Noble County Courthouse, 101 N. Orange St., Albion, Indiana 46701 on Monday, June 25, 2018 at 9:00 am EST. Bidders may submit bids on any or all packages, however, the bid for each package should be sealed in a minimum size envelope of 9”x12” and shall bear the name of the bidder and the Bid Package number for the respective bid on the outside of the envelope. All bidders must furnish with their bids a Bid Bond or Certified Check equal to 10% of the total bid payable to the Noble County Board of Commissioners. A combination Bid/Performance Bond equal to 100% of the total price will be acceptable. All bids shall be on the appropriate forms, completely sign and filled out and bound with the contract documents. -

Distribution and Diversity of Freshwater Crabs (Decapoda: Brachyura: Potamidae, Gecarcinucidae) in Iranian Inland Waters Ardavan Farhadi , Muzaffer Mustafa Harlıoğlu
EISSN 2602-473X AQUATIC SCIENCES AND ENGINEERING Aquat Sci Eng 2018; 33(4): 110-116 • DOI: 10.26650/ASE2018422064 Review Distribution and Diversity of Freshwater Crabs (Decapoda: Brachyura: Potamidae, Gecarcinucidae) in Iranian Inland Waters Ardavan Farhadi , Muzaffer Mustafa Harlıoğlu Cite this article as: Farhadi, A., Harlıoğlu, M.M. (2018). Distribution and Diversity of Freshwater Crabs (Decapoda: Brachyura: Potamidae, Gec- arcinucidae) in Iranian Inland Waters. Aquatic Sciences and Engineering, 33(4): 110-116. ABSTRACT This article reviews the current knowledge of primary freshwater crabs (Decapoda, Brachyura) in Iranian inland waters, with the purpose of classifying the exact number of species, the threat sta- tus, and their distribution and diversity. Previous studies have reported that Iranian inland waters have eight freshwater crab species and there was no accurate information on the distribution of freshwater crab species in Iran. This review article describes that an additional six freshwater crab species, Potamon gedrosianum, P. magnum, P. mesopotamicum, P. ilam, Sodhiana blanfordi, and S. iranica, are also present in Iran. Therefore, there are 14 freshwater crab species currently known in Iran, which belong to two families (Gecarcinucidae and Potamidae). The genus Potamon is rep- resented by 11 species, and the genus Sodhiana is represented by 3 species (found in south and south east of Iran). In addition, this review presents a distribution map and the possible threats for each species. Keywords: Brachyura, decapoda, freshwater crabs, distribution, Iran INTRODUCTION ter swamps, stagnant ponds and rice fields, and even in tree hollows and leaf axils (Yeo et al., Primary freshwater crabs (Yeo et al., 2008, 2012) 2008; Cumberlidge et al., 2009). -

Freshwater Crabs As Predators and Prey: the Case of Ptychophallus Uncinatus Campos & Lemaitre, 1999 (Brachyura, Pseudothelphusidae) from Costa Rica, Central America
Latin American Journal of Aquatic Research, 47(1): 18Ptychophallus-26, 2019 uncinatus from Costa Rica 1 DOI: 10.3856/vol47-issue1-fulltext-3 Research Article Freshwater crabs as predators and prey: the case of Ptychophallus uncinatus Campos & Lemaitre, 1999 (Brachyura, Pseudothelphusidae) from Costa Rica, Central America Ingo S. Wehrtmann1,2, Dayan Hernández-Díaz3 & Neil Cumberlidge4 1Museo de Zoología, Escuela de Biología, Universidad de Costa Rica, San José, Costa Rica 2Unidad de Investigación Pesquera y Acuicultura (UNIP), Centro de Investigación en Ciencias del Mar y Limnología (CIMAR), Universidad de Costa Rica, San José, Costa Rica 3Escuela de Biología, Universidad Latina de Costa Rica, Sede San Pedro, Costa Rica 4Department of Biology, Northern Michigan University, Marquette, MI, USA Corresponding author: Ingo S. Wehrtmann ([email protected]) ABSTRACT. Primary freshwater crabs are an important component of the food web in aquatic ecosystems, but our knowledge about the role of these decapods as predators and as prey is far from complete. Here we report observations of the feeding habits of the pseudothelphusid crab Ptychophallus uncinatus Campos & Lemaitre, 1999, made in 2013 during exploratory observations after sunset in the dusk and darkness of the early evening within the Veragua Rainforest Research & Adventure Park, Limón Province, in the Atlantic drainage of Costa Rica. We observed a case of cannibalism where an adult P. uncinatus was feeding on a smaller crab. Furthermore, P. uncinatus was observed to prey on an insect larva, a frog, and a lizard on three separate occasions. Additionally, a spider of the family Ctenidae was discovered feeding on a specimen of P. -

Decapoda, Brachyura
APLICACIÓN DE TÉCNICAS MORFOLÓGICAS Y MOLECULARES EN LA IDENTIFICACIÓN DE LA MEGALOPA de Decápodos Braquiuros de la Península Ibérica bérica I enínsula P raquiuros de la raquiuros B ecápodos D de APLICACIÓN DE TÉCNICAS MORFOLÓGICAS Y MOLECULARES EN LA IDENTIFICACIÓN DE LA MEGALOPA LA DE IDENTIFICACIÓN EN LA Y MOLECULARES MORFOLÓGICAS TÉCNICAS DE APLICACIÓN Herrero - MEGALOPA “big eyes” Leach 1793 Elena Marco Elena Marco-Herrero Programa de Doctorado en Biodiversidad y Biología Evolutiva Rd. 99/2011 Tesis Doctoral, Valencia 2015 Programa de Doctorado en Biodiversidad y Biología Evolutiva Rd. 99/2011 APLICACIÓN DE TÉCNICAS MORFOLÓGICAS Y MOLECULARES EN LA IDENTIFICACIÓN DE LA MEGALOPA DE DECÁPODOS BRAQUIUROS DE LA PENÍNSULA IBÉRICA TESIS DOCTORAL Elena Marco-Herrero Valencia, septiembre 2015 Directores José Antonio Cuesta Mariscal / Ferran Palero Pastor Tutor Álvaro Peña Cantero Als naninets AGRADECIMIENTOS-AGRAÏMENTS Colaboración y ayuda prestada por diferentes instituciones: - Ministerio de Ciencia e Innovación (actual Ministerio de Economía y Competitividad) por la concesión de una Beca de Formación de Personal Investigador FPI (BES-2010- 033297) en el marco del proyecto: Aplicación de técnicas morfológicas y moleculares en la identificación de estados larvarios planctónicos de decápodos braquiuros ibéricos (CGL2009-11225) - Departamento de Ecología y Gestión Costera del Instituto de Ciencias Marinas de Andalucía (ICMAN-CSIC) - Club Náutico del Puerto de Santa María - Centro Andaluz de Ciencias y Tecnologías Marinas (CACYTMAR) - Instituto Español de Oceanografía (IEO), Centros de Mallorca y Cádiz - Institut de Ciències del Mar (ICM-CSIC) de Barcelona - Institut de Recerca i Tecnología Agroalimentàries (IRTA) de Tarragona - Centre d’Estudis Avançats de Blanes (CEAB) de Girona - Universidad de Málaga - Natural History Museum of London - Stazione Zoologica Anton Dohrn di Napoli (SZN) - Universitat de Barcelona AGRAÏSC – AGRADEZCO En primer lugar quisiera agradecer a mis directores, el Dr.