The Impact of Grape Ripeness Level on Berry and Wine Composition and Potential Wine Style of Vitis Vinifera L
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Growing Vitis Vinifera In
Report of research sponsored by the New York State Wine and Grape Foundation hi(T Produced by Communications Services NYS Agricultural Experiment Station Cornell University • Geneva GROWING VITIS VINIFERA GRAPES IN NEW YORK STATE I - Performance of New and Interesting Varieties WRITTEN BY Robert M. Pool 1, Gary E. Howard2, Richard Dunst3, John Dyson4, Thomas Henick-Kling5, Jay Freer 6, Larry Fuller-Perrine 7, Warren Smith8 and Alice Wise 9 1 Professor of Viticulture, Department of Horticultural Sciences, Cornell University, New York State Agricultural Experiment Station, Geneva, NY 2 Research Support Specialist, Department of Horticultural Sciences, Cornell University, New York State Agricultural Experiment Station, Geneva, NY 3 Research Support Specialist, Department of Horticultural Sciences, Cornell University, New York State Agricultural Experiment Station, Fredonia, NY • Veraison Wine Cellars, Millbrook, NY. 'Assistant Professor of Enology, Department of Food Science and Technology, Cornell University, New York State Agricultural Experiment Station, Geneva, NY 6 Administrative Manager, Department of Horticultural Sciences, Cornell University, New York State Agricultural Experiment Station, Geneva, NY 7 Research Support Specialist, Department of Horticultural Sciences, Cornell University, New York State Agricultural Experiment Station, Riverhead, NY 8 Cornell Cooperative Extension, illster County, Highland, NY °Cornell Cooperative Extension, Suffolk County, Riverhead, NY CONTENTS FoREWORD ...........................................................................................i -

Wine of Origin Booklet
Version 20101201 TABLE OF CONTENTS Introduction ....................................................................................................................................... 3 Wine and Spirit Board ........................................................................................................................ 3 Composition ....................................................................................................................................... 3 Functions ............................................................................................................................................ 3 Operation ........................................................................................................................................... 4 Wine of Origin Scheme ...................................................................................................................... 6 Importance of Origin .......................................................................................................................... 6 Demarcation of areas of Origin .......................................................................................................... 6 Criteria for the demarcation of areas of Origin ................................................................................. 7 Geographical unit ............................................................................................................................... 8 The role of cultivar in Wine of Origin ................................................................................................ -

Canada-EU Wine and Spirits Agreement
6.2.2004 EN Official Journal of the European Union L 35/3 AGREEMENT between the European Community and Canada on trade in wines and spirit drinks THE EUROPEAN COMMUNITY, hereafter referred to as ‘the Community', and CANADA, hereafter jointly referred to as ‘the Contracting Parties', RECOGNISING that the Contracting Parties desire to establish closer links in the wine and spirits sector, DESIROUS of creating more favourable conditions for the harmonious development of trade in wine and spirit drinks on the basis of equality and mutual benefit, HAVE AGREED AS FOLLOWS: TITLE I — ‘labelling' shall mean any tag, brand, mark, pictorial or other descriptive matter, written, printed, stencilled, marked, embossed or impressed on, or attached to, a INITIAL PROVISIONS container of wine or a spirit drink, Article 1 — ‘WTO Agreement' refers to the Marrakesh Agreement establishing the World Trade Organisation, Objectives 1. The Contracting Parties shall, on the basis of — ‘TRIPs Agreement' refers to the Agreement on trade-related non-discrimination and reciprocity, facilitate and promote aspects of intellectual property rights, which is contained trade in wines and spirit drinks produced in Canada and the in Annex 1C to the WTO Agreement, Community, on the conditions provided for in this Agreement. 2. The Contracting Parties shall take all reasonable measures — ‘1989 Agreement' refers to the Agreement between the to ensure that the obligations laid down in this Agreement are European Economic Community and Canada concerning fulfilled and that the objectives set out in this Agreement are trade and commerce in alcoholic beverages concluded on attained. 28 February 1989. Article 2 2. -
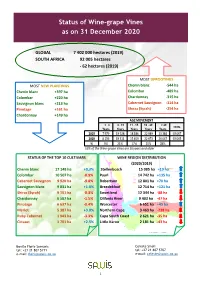
Status of Wine-Grape Vines As on 31 December 2020
Status of Wine-grape Vines as on 31 December 2020 GLOBAL 7 402 000 hectares (2019) SOUTH AFRICA 92 005 hectares - 62 hectares (2019) MOST UPROOTINGS MOST NEW PLANTINGS Chenin blanc -544 ha Chenin blanc +397 ha Colombar -489 ha Colombar +220 ha Chardonnay -315 ha Sauvignon blanc +213 ha Cabernet Sauvignon -314 ha Pinotage +161 ha Shiraz (Syrah) -254 ha Chardonnay +149 ha AGE MOVEMENT < 4 4 - 10 11 - 15 16 - 20 > 20 TOTAL Years Years Years Years Years 2019 7 979 19 528 18 186 22 989 23 384 92 067 2020 8 156 19 311 15 819 22 673 26 047 92 005 % 9% 21% 17% 25% 28% 53% of the Wine-grape Vines are 16 years and older STATUS OF THE TOP 10 CULTIVARS WINE REGION DISTRIBUTION (2020/2019) Chenin blanc 17 148 ha +0,3% Stellenbosch 15 085 ha +19 ha Colombar 10 507 ha -0.9% Paarl 14 742 ha +135 ha Cabernet Sauvignon 9 920 ha -9.8% Robertson 12 801 ha +70 ha Sauvignon blanc 9 831 ha +1.8% Breedekloof 12 714 ha +121 ha Shiraz (Syrah) 9 151 ha -0.3% Swartland 12 344 ha -88 ha Chardonnay 6 587 ha -1.5% Olifants River 9 403 ha -47 ha Pinotage 6 637 ha -0.4% Worcester 6 651 ha +45 ha Merlot 5 387 ha +3.0% Northern Cape 3 463 ha -238 ha Ruby Cabernet 1 943 ha -3.3% Cape South Coast 2 621 ha -35 ha Cinsaut 1 701 ha +2.5% Little Karoo 2 181 ha -43 ha Bonita Floris-Samuels Celeste Small tel: +27 21 807 5711 tel: +27 21 807 5707 e-mail: [email protected] e-mail: [email protected] 1 Statistics i.r.o. -

Wine from Cold Hardy Grapes
Making, Blending, and Selling Wines From Cold Hardy Cultivars Stephen Menke Colorado State University-WCRC Desirable Traits in Hybrid Grape Wines Great fruitiness Usually good color Sufficient acid Great taste intensity upon presentation to mouth Good food pairing Good dry or sweet Problem Traits in Hybrid Grape Wines Some strong varietal aromas and tastes Can be too acid Tannins low Prone to structural breakdown of flavor and body Sweeter wines prone to re-fermentation Cool Climate Vitis vinifera Intraspecific Crosses Cool Climate = winter minimum of -50F to -150F, and depends on acclimation Lemberger (red, moderate cold resistance, fruity, good wine quality) Comtessa (red used for white, moderate cold resistance, fruity wine) Siegerrebe (white, fairly cold resistant, very floral wine) Noblessa (white, moderate cold resistance, good wine quality reported) Morio muscat (white, moderate cold, northeast US, very floral and fruity) Madeleine Angevine (white, moderate cold, good wine quality reported) Cool Climate Hybrids/Natives vinifera/American, vinifera/amurensis, Cornell, Minnesota, UC Davis Useful site http://viticulture.hort.iastate.edu/cultivars/cultivars.html Cool Climate = winter minimum of -50F to -150F, and depends on acclimation Reds Baco noir, Chambourcin, Chancellor, Concord, Corot noir, Crimson cabernet, DeChaunac, GR7, Kozma 55, Kozma 525, Landot noir, Leon Millot, Marechal Foch, Noiret, Norton, St. Vincent Cool Climate Hybrids/Natives vinifera/American, vinifera/amurensis, Cornell, Minnesota, UC Davis Cool Climate = winter minimum of -50F to -150F, and depends on acclimation Whites Catawba (rosé), Cayuga white, Chardonel, Delaware, Niagara, Seyval blanc, Traminette, Valvin muscat, Veeblanc, Vidal blanc, Vignoles Cold Climate Hybrids Swenson, Minnesota, Cornell, etc. Cold Climate = winter minimum of -150F to - 300F, depends on acclimation Reds Baltica, Frontenac, Marquette, MN 1200, Sabrevois, St. -

THE DEVELOPMENT TEAM Principal Investigator Prof
Paper 05: Food and Beverage Service Module 20: Wines of Germany THE DEVELOPMENT TEAM Principal Investigator Prof. S. P. Bansal Vice chancellor , Indira Gandhi University, Meerpur, Rewari, Haryana Co-Principal Investigator Dr. Prashant K. Gautam, Director, UIHTM, Panjab University, Chandigarh Paper Coordinator Dr. Neeraj Aggarwal UIHTM, Panjab University, Chandigarh Content Writer Mahendra Singh Negi Faculty of Hospitality Management Amrapali Group of Institutes Content Reviewer Prof .S.K Gupta Dean, CMTHS HNB Garhwal University, Srinagar ITEMS DESCRIPTION OF MODULE Paper Name Food and Beverage Service Module Name/Title Wines from Germany Pre requisites Wine manufacturing process and purpose of grapes in wine making. Objectives Students will be able to understand the major wine producing regions of Germany along with the types of grapes used in manufacturing German wine along with the various wine appreciation used in Germany Keywords Sekt, Hock, Mosel TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. Wine Laws 4. Sekt 5. Wine Producing Region 6. Grape Variety 7. Reading A Wine Label 8. German Wine Terms 9. Summary QUADRANT 1 1. Learning outcome This chapter introduces the students with the wines from Germany covering wine producing regions, grape varieties, wine laws/wine appreciation/wine quality control terms and other wine terms specifically based for German wines. On completion of this chapter, the students should be able to: Understand the various wine producing regions of Germany Major grape varieties of Germany Wine laws /appreciation of Germany Wine terms used for German wines 2. Introduction Germany takes sixth place among the wine producing nations of the world. Much of the production is intended for export, while Germany itself imports huge volumes of wines. -

OIV List of Grape Vine Varieties and Their Synonyms (Australia)
OIV list of grape vine varieties and their synonyms (Australia) Prime Name Synonyms Abouriou Aglianico Albariño Albarino Alvarinho Albillo Aléatico Alfrocheiro Alicante Bouschet Alicante Henri Bouschet Aligoté Alphonse Lavallée Alvarelhao Ancellotta Ansonica Inzolia Insolia Aranel Arinto Arneis Assyrtiko Azal Aubun Auxerrois Aucerot Barbera Bastardo Trousseau Bianco d'Alessano Biancone Black Corinth Zante Currant Blaufrankisch Blaufränkisch Blaufraenkisch Limberger/Lemberger Boal Branco Malvasia Fina Bonvedro Parraleta Carcajolo Nero Page 1 of 8 October 2017 Wine Australia OIV list of grape vine varieties and their synonyms (Australia) Tinta Caiada Bourboulenc Brachetto Brown Frontignac Brown Muscat Muscat à petits grains rouges Burger Cabernet Franc Cabernet Sauvignon Cabernet Canaiolo Cañocazo Cardinal Carignan Carignane Mazuela Carina Carmenère Carnelian Cascade Cesanese Chambourcin Chardonnay Chardonnay Musque Chasselas Chenin Blanc Cienna Cinsaut Oeillade Blue Imperial Clairette Blanquette Colombard Colorino Cortese Corvina Counoise Crouchen Crystal Crystall Crystil Krystal Cygne Blanc Page 2 of 8 Wine Australia OIV list of grape vine varieties and their synonyms (Australia) Dolcetto Doradillo Cayetana Blanca Dourado Galego Dourado Durif Petite Sirah Ehrenfelser Emerald Riesling Falanghina Farana Damaschino Planta Fina de Pedralba Fernao Pires Fiano Flora Folle Blanche Fortana Freisa Frontignac Moscato Bianco Muscat Blanc Muscat à petits grains blancs White Frontignac Furmint Gamay Garganega Gewurztraminer Gewürztraminer Red Traminer -

Wine and Juice Grape Varieties for Cool Climates
Acknowledgments This publication is based on New Yorl< Food and Life Sciences Bulletin 80, July 1779, Grape Varieties for New York State. The authors are indebted to their predecessors, John Einset (deceased), John Watson (deceased),Willard Robinson, Contents and Iceit11 Kimball, who developed the earlier bulletin and generated much of the information contained therein. Gary Introduction 2 Howard's expertise was invaluable to the Leading American Varieties 3 Cornell evaluations of Vitis uitzifera varieties, and Ben Gavitt and Joe Bertino American Varieties of Limited Acreage 3 (deceased) provided their skillful wine- malting expertise. Phillip McInnis, Jr., French-American and Other Interspecific Varieties 4 assisted with field evaluations and generated the early variety data bases Interspecific Varieties for Red Wine Production 4 upon which this publication was based. I~lterspecificVarieties for White Wine Production 5 Raymond Mallaber and the Field Research Unit of Geneva, as well as the Recent Introductions of Interspecific Hybrids staff of the Vineyard Laboratory of Fredonia, supplied skilled and enthusias- Cold-Hardy Varieties Developed by tic viticult~~ralexpertise. James Kamas Elmer Swenson, Osceola, Wisconsin and Nelson Shaulis provided thoughtf~ll reviews of this manuscript. We are European (Vitis uinife~a)Wine Grape Varieties grateful for financial support from the Leading Vitis uirzifem White Wine Varieties New Yorl< State Grape 1)roduction Research Fund, the New Yorl< Wine and Other Vitis vi~ziferaWhite Wine Varieties Grape Foundation, and John Dyson. To all of the above, as well as to many others Leading Vitis uinifera Red Wine Varieties who contributed in significant ways, the Vitis uinifera authors express their gratitude and Other Red Wine Varieties appreciation. -

Chemical Composition of Antioxidant Dietary Fiber of Grape Skins From
1 Chemical composition of dietary fiber and polyphenols of five different varieties of wine 2 grape pomace skins 3 4 Qian Deng, Michael H. Penner, Yanyun Zhao* 5 6 7 Department of Food Science and Technology, 100 Wiegand Hall, 8 Oregon State University, Corvallis, OR 97331, USA 9 10 11 12 13 14 15 16 17 *To whom all correspondence should be sent: 18 Dr. Yanyun Zhao, Professor 19 Department of Food Science and Technology 20 Oregon State University 21 100 Wiegand Hall 22 Corvallis, OR 97331-6602 23 Tel: (541) 737-9151 24 Fax: (541) 737-1877 25 E-mail: [email protected] 1 1 Abstract 2 The skins of two white wine grape pomace (WWGP) and three red wine grape pomace 3 (RWGP) from US Pacific Northwest were analyzed for their dietary fiber (DF) and phenolics 4 composition. DF was measured by gravimetric-enzymatic method with sugar profiling by HPLC- 5 ELSD. Insoluble DF composed of Klason lignin (7.9-36.1% DM), neutral sugars (4.9-14.6% 6 DM), and uronic acid (3.6-8.5% DM) weighed more than 95.5% of total DF in all five WGP 7 varieties. WWGP was significantly lower in DF (17.3-28.0% DM) than those of RWGP (51.1- 8 56.3%), but extremely higher in soluble sugar (55.8-77.5% DM vs. 1.3-1.7% DM) (p<0.05). 9 Soluble polyphenols were extracted by acidified 70% acetone and measured 10 spectrophotometrically. Compared with WWGP, RWGP had higher values in total phenolics 11 content (21.4-26.7 mg GAE/g DM vs. -
The Potential of Grape Pomace Varieties As a Dietary Source of Pectic Substances
foods Review The Potential of Grape Pomace Varieties as a Dietary Source of Pectic Substances Mariana Spinei and Mircea Oroian * Department of Food Technologies, Food Production and Environment Safety, Faculty of Food Engineering, Stefan cel Mare University of Suceava, 720229 Suceava, Romania; mariana.spinei@fia.usv.ro * Correspondence: m.oroian@fia.usv.ro Abstract: Grape pomace is one of the most abundant solid by-products generated during winemaking. A lot of products, such as ethanol, tartrates, citric acid, grape seed oil, hydrocolloids, bioactive compounds and dietary fiber are recovered from grape pomace. Grape pomace represents a major interest in the field of fiber extraction, especially pectin, as an alternative source to conventional ones, such as apple pomace and citrus peels, from which pectin is obtained by acid extraction and precipitation using alcohols. Understanding the structural and functional components of grape pomace will significantly aid in developing efficient extraction of pectin from unconventional sources. In recent years, natural biodegradable polymers, like pectin has invoked a big interest due to versatile properties and diverse applications in food industry and other fields. Thus, pectin extraction from grape pomace could afford a new reason for the decrease of environmental pollution and waste generation. This paper briefly describes the structure and composition of grape pomace of different varieties for the utilization of grape pomace as a source of pectin in food industry. Keywords: grape pomace; grape skin; grape seeds; grape inflorescence architectures; pectin; extraction Citation: Spinei, M.; Oroian, M. The 1. Introduction Potential of Grape Pomace Varieties The main by-products of the wine industry are grape pomace, grape seeds, grape as a Dietary Source of Pectic bunches, yeast and tartrates sediments [1]. -

Chemical Composition of Dietary Fiber and Polyphenols of Wine Grape Pomace Skins and Development of Wine Grape (Cv
AN ABSTRACT OF THE THESIS OF Qian Deng for the degree of Master of Science in Food Science and Technology presented on February 11, 2011. Title: Chemical Composition of Dietary Fiber and Polyphenols of Wine Grape Pomace Skins and Development of Wine Grape (cv. Merlot) Pomace Extract Based Films. Abstract approved: _____________________________________________________________________ Yanyun Zhao The objectives of this project were to investigate the chemical composition of five varieties of wine grape pomace (WGP) skins obtained in the Pacific Northwest of the United States with the emphasis on dietary fiber (DF) and polyphenolic compounds, and further to evaluate the feasibility of developing WGP extract based edible films in terms of their physicochemical, nutritional and antimicrobial properties. Research studied two varieties of white WGP (WWGP) skins (vinifera L. cv. Morio Muscat and cv. Muller Thurgau) and three varieties of red WGP (RWGP) skins (Vitis vinifera L. cv. Cabernet Sauvignon, cv. Pinot Noir and cv. Merlot). DF was measured by gravimetric-enzymatic method with sugar profiling by HPLC-ELSD. Soluble polyphenols were extracted by 70% acetone/0.1% HCl/29.9% deionized water and measured spectrophotometrically. The major composition of insoluble DF (IDF) were Klason lignin (7.9-36.1% DM), neutral sugars (4.9-14.6% DM), and uronic acid (3.6-8.5% DM), which weighed more than 95.1% of total DF in all five WGP varieties. RWGP was significantly higher in DF (51.1-56.3% DM) than those of WWGP (17.3-28.0% DM), but lower in soluble sugar (1.3-1.7% DM in RWGP vs. -

Final Report Guidelines
Enhanced varieties and clones to meet the challenges of climate change and deliver lower alcohol wines FINAL REPORT to AUSTRALIAN GRAPE AND WINE AUTHORITY Project Number: CSP 0902 Principal Investigator: Peter Clingeleffer Research Organisation: CSIRO Date: 15/05/2015 Enhanced varieties and clones to meet the challenges of climate change and deliver lower alcohol wines Peter. R. Clingeleffer and Hilary P. Davis CSIRO Agriculture, PMB 2 Glen Osmond, SA 5065. Date: 15/05/2015 Publisher: CSIRO Agriculture Copyright and Disclaimer: © 2015 CSIRO. To the extent permitted by law, all rights are reserved and no part of this publication covered by copyright may be reproduced or copied in any form or by any means except with the written permission of CSIRO. Important Disclaimer: CSIRO advises that the information contained in this publication comprises general statements based on scientific research. The reader is advised and needs to be aware that such information may be incomplete or unable to be used in any specific situation. No reliance or actions must therefore be made on that information without seeking prior expert professional, scientific and technical advice. To the extent permitted by law, CSIRO (including its employees and consultants) exclude all liability to any person for any consequences, including but not limited to all losses, damages, costs, expenses and any other compensation, arising directly or indirectly from using this publication (in part or in whole) and any information or material contained in it. Acknowledgements: The authors of this report wish to acknowledge the financial support from the Commonwealth under the “Australia’s Farming Future: Climate Change Research program” made possible through the Australian Grape and Wine Authority, the investment body of Australia’s grape growers and winemakers.