Terpenes in the Aroma of Grapes and Wines: a Review
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Growing Vitis Vinifera In
Report of research sponsored by the New York State Wine and Grape Foundation hi(T Produced by Communications Services NYS Agricultural Experiment Station Cornell University • Geneva GROWING VITIS VINIFERA GRAPES IN NEW YORK STATE I - Performance of New and Interesting Varieties WRITTEN BY Robert M. Pool 1, Gary E. Howard2, Richard Dunst3, John Dyson4, Thomas Henick-Kling5, Jay Freer 6, Larry Fuller-Perrine 7, Warren Smith8 and Alice Wise 9 1 Professor of Viticulture, Department of Horticultural Sciences, Cornell University, New York State Agricultural Experiment Station, Geneva, NY 2 Research Support Specialist, Department of Horticultural Sciences, Cornell University, New York State Agricultural Experiment Station, Geneva, NY 3 Research Support Specialist, Department of Horticultural Sciences, Cornell University, New York State Agricultural Experiment Station, Fredonia, NY • Veraison Wine Cellars, Millbrook, NY. 'Assistant Professor of Enology, Department of Food Science and Technology, Cornell University, New York State Agricultural Experiment Station, Geneva, NY 6 Administrative Manager, Department of Horticultural Sciences, Cornell University, New York State Agricultural Experiment Station, Geneva, NY 7 Research Support Specialist, Department of Horticultural Sciences, Cornell University, New York State Agricultural Experiment Station, Riverhead, NY 8 Cornell Cooperative Extension, illster County, Highland, NY °Cornell Cooperative Extension, Suffolk County, Riverhead, NY CONTENTS FoREWORD ...........................................................................................i -

Changes in the Aromatic Compounds Content in the Muscat Wines As a Result of the Application of Ultrasound During Pre-Fermentative Maceration
foods Article Changes in the Aromatic Compounds Content in the Muscat Wines as a Result of the Application of Ultrasound during Pre-Fermentative Maceration Fátima Aragón-García, Ana Ruíz-Rodríguez * and Miguel Palma Department of Analytical Chemistry, Center of Agri-Food and Wine Research (IVAGRO), Faculty of Science, University of Cadiz, 11510 Puerto Real, Spain; [email protected] (F.A.-G.); [email protected] (M.P.) * Correspondence: [email protected] Abstract: This research focuses on the aromatic composition of Muscat of Alexandria wines after the application of ultrasound for 40 or 80 min during a 4 h pre-fermentative maceration process. Two methods of ultrasound application were compared in this study: probe ultrasound and bath ultrasound, for periods of 10–20 min per hour. Increases of more than 200% were obtained for some of the compounds from the skins, such as two of its terpenes, citronellol and nerol. On the other hand, increases in alcohol and ester values were registered with the application of ultrasound for 40 min. However, a significant decrease in these compounds was recorded when the ultrasound process was extended. In fact, when ultrasound was applied for 80 min, content values were even lower than those registered for the wine produced without the application of ultrasound. At the sensory level, the effect resulting from probe and bath ultrasound application for different times were compared, where most of the judges successfully discriminated the wines resulting from the application of ultrasound bath. According to data, the wines resulting from the application of ultrasound bath for Citation: Aragón-García, F.; 80 min presented the most significant differences, which affected the aromas of white fruit, tropical Ruíz-Rodríguez, A.; Palma, M. -

Effect of Short Ageing on Lees on the Mannoprotein Content, Aromatic
1 1 Effect of short ageing on lees on the mannoprotein content, aromatic 2 profile and sensorial character of white wines 3 4 Marta Juega, Alfonso V. Carrascosa and Adolfo J. Martinez-Rodriguez* 5 6 Institute of Food Science Research (CIAL), CSIC-UAM, Department of 7 Biotechnology and Microbiology. C/ Nicolás Cabrera, 9. Cantoblanco Campus, 8 Autónoma University of Madrid, 28049, Madrid, Spain 9 10 *Corresponding author: 11 Tel: +34 91 001 79 64 12 Fax: +34 91 001 79 05 13 E-mail address: [email protected] 14 15 16 17 18 19 20 21 22 23 24 2 25 26 Abstract 27 28 In Albariño white wines, aging of wines on lees is a technique not used or only 29 used empirically by some producers to obtain a distinctive character in the final 30 wine. This study analyzes the influence of a short aging on lees on the chemical 31 and sensorial parameters of this young white wine. Albariño grape must was 32 inoculated with a locally selected yeast (S. cerevisiae 1) and the effect of a 33 short aging on lees was studied during different times (10, 20, 30, 40 and 50 34 days). Mannoprotein content and the aromatic profile were determined and a 35 sensorial analysis of the wines was conducted. Results showed that aging time 36 was correlated with the concentration of some key aroma compounds and 37 mannoproteins in Albariño wines. The best sensorial character was obtained in 38 wines aged 20 days on lees. Further aging times decreased the sensorial 39 quality of Albariño wine and modified its volatile profile and mannoprotein 40 concentration. -

Wine of Origin Booklet
Version 20101201 TABLE OF CONTENTS Introduction ....................................................................................................................................... 3 Wine and Spirit Board ........................................................................................................................ 3 Composition ....................................................................................................................................... 3 Functions ............................................................................................................................................ 3 Operation ........................................................................................................................................... 4 Wine of Origin Scheme ...................................................................................................................... 6 Importance of Origin .......................................................................................................................... 6 Demarcation of areas of Origin .......................................................................................................... 6 Criteria for the demarcation of areas of Origin ................................................................................. 7 Geographical unit ............................................................................................................................... 8 The role of cultivar in Wine of Origin ................................................................................................ -

Investigating the Aroma of Syrah Wines from the Northern Rhone Valley Using Supercritical CO2-Dearomatized Wine As a Matrix for Reconstitution Studies
Open Archive Toulouse Archive Ouverte OATAO is an open access repository that collects the work of Toulouse researchers and makes it freely available over the web where possible This is an author’s version published in: http://oatao.univ-toulouse.fr/27301 Official URL DOI : https://doi.org/10.1021/acs.jafc.0c04328 To cite this version: Geffroy, Olivier and Morère, Marie and Lopez, Ricardo and Pasquier, Grégory and Condoret, Jean- Stéphane Investigating the Aroma of Syrah Wines from the Northern Rhone Valley Using Supercritical CO2-Dearomatized Wine as a Matrix for Reconstitution Studies. (2020) Journal of Agricultural and Food Chemistry, 68 (41). 11512-11523. ISSN 0021-8561 Any correspondence concerning this service should be sent to the repository administrator: [email protected] Investigating the Aroma of Syrah Wines from the Northern Rhone ‑ Valley Using Supercritical CO2 Dearomatized Wine as a Matrix for Reconstitution Studies Olivier Geffroy,* Marie Morere,̀ Ricardo Lopez, Gregorý Pasquier, and Jean-Stephané Condoret ABSTRACT: This study aimed to investigate the key compounds involved in the aroma of French Syrah wines from the northern Rhone valley from two vintages characterized by distinct climatic conditions. The volatile composition of the wines was assessed through the determination of 76 molecules. After identifying the best matrix and best model for aroma reconstitution studies, omission tests were conducted using the Pivot profile method. For both vintages, 35 molecules with odor activity values (OAVs) above 0.5 were identified. While remarkably high levels of 2-furfurylthiol (FFT) were reported in both wines, rotundone and 3- sulfanylhexanol (3SH) enabled the strongest discrimination between the two wines. -

Canada-EU Wine and Spirits Agreement
6.2.2004 EN Official Journal of the European Union L 35/3 AGREEMENT between the European Community and Canada on trade in wines and spirit drinks THE EUROPEAN COMMUNITY, hereafter referred to as ‘the Community', and CANADA, hereafter jointly referred to as ‘the Contracting Parties', RECOGNISING that the Contracting Parties desire to establish closer links in the wine and spirits sector, DESIROUS of creating more favourable conditions for the harmonious development of trade in wine and spirit drinks on the basis of equality and mutual benefit, HAVE AGREED AS FOLLOWS: TITLE I — ‘labelling' shall mean any tag, brand, mark, pictorial or other descriptive matter, written, printed, stencilled, marked, embossed or impressed on, or attached to, a INITIAL PROVISIONS container of wine or a spirit drink, Article 1 — ‘WTO Agreement' refers to the Marrakesh Agreement establishing the World Trade Organisation, Objectives 1. The Contracting Parties shall, on the basis of — ‘TRIPs Agreement' refers to the Agreement on trade-related non-discrimination and reciprocity, facilitate and promote aspects of intellectual property rights, which is contained trade in wines and spirit drinks produced in Canada and the in Annex 1C to the WTO Agreement, Community, on the conditions provided for in this Agreement. 2. The Contracting Parties shall take all reasonable measures — ‘1989 Agreement' refers to the Agreement between the to ensure that the obligations laid down in this Agreement are European Economic Community and Canada concerning fulfilled and that the objectives set out in this Agreement are trade and commerce in alcoholic beverages concluded on attained. 28 February 1989. Article 2 2. -
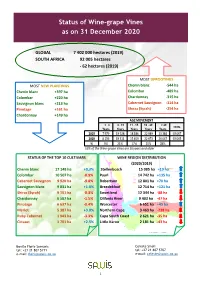
Status of Wine-Grape Vines As on 31 December 2020
Status of Wine-grape Vines as on 31 December 2020 GLOBAL 7 402 000 hectares (2019) SOUTH AFRICA 92 005 hectares - 62 hectares (2019) MOST UPROOTINGS MOST NEW PLANTINGS Chenin blanc -544 ha Chenin blanc +397 ha Colombar -489 ha Colombar +220 ha Chardonnay -315 ha Sauvignon blanc +213 ha Cabernet Sauvignon -314 ha Pinotage +161 ha Shiraz (Syrah) -254 ha Chardonnay +149 ha AGE MOVEMENT < 4 4 - 10 11 - 15 16 - 20 > 20 TOTAL Years Years Years Years Years 2019 7 979 19 528 18 186 22 989 23 384 92 067 2020 8 156 19 311 15 819 22 673 26 047 92 005 % 9% 21% 17% 25% 28% 53% of the Wine-grape Vines are 16 years and older STATUS OF THE TOP 10 CULTIVARS WINE REGION DISTRIBUTION (2020/2019) Chenin blanc 17 148 ha +0,3% Stellenbosch 15 085 ha +19 ha Colombar 10 507 ha -0.9% Paarl 14 742 ha +135 ha Cabernet Sauvignon 9 920 ha -9.8% Robertson 12 801 ha +70 ha Sauvignon blanc 9 831 ha +1.8% Breedekloof 12 714 ha +121 ha Shiraz (Syrah) 9 151 ha -0.3% Swartland 12 344 ha -88 ha Chardonnay 6 587 ha -1.5% Olifants River 9 403 ha -47 ha Pinotage 6 637 ha -0.4% Worcester 6 651 ha +45 ha Merlot 5 387 ha +3.0% Northern Cape 3 463 ha -238 ha Ruby Cabernet 1 943 ha -3.3% Cape South Coast 2 621 ha -35 ha Cinsaut 1 701 ha +2.5% Little Karoo 2 181 ha -43 ha Bonita Floris-Samuels Celeste Small tel: +27 21 807 5711 tel: +27 21 807 5707 e-mail: [email protected] e-mail: [email protected] 1 Statistics i.r.o. -

Aromatic Characterization of New White Wine Varieties Made from Monastrell Grapes Grown in South-Eastern Spain
molecules Article Aromatic Characterization of New White Wine Varieties Made from Monastrell Grapes Grown in South-Eastern Spain Juan Daniel Moreno-Olivares, Maria José Giménez-Bañón, Diego Fernando Paladines-Quezada , Jose Cayetano Gómez-Martínez, Ana Cebrián-Pérez, Jose Ignacio Fernández-Fernández, Juan Antonio Bleda-Sánchez and Rocio Gil-Muñoz * Instituto Murciano de Investigación y Desarrollo Agrario y Alimentario (IMIDA), Ctra. La Alberca s/n, 30150 Murcia, Spain; [email protected] (J.D.M.-O.); [email protected] (M.J.G.-B.); [email protected] (D.F.P.-Q.); [email protected] (J.C.G.-M.); [email protected] (A.C.-P.); [email protected] (J.I.F.-F.); [email protected] (J.A.B.-S.) * Correspondence: [email protected] Received: 1 July 2020; Accepted: 25 August 2020; Published: 27 August 2020 Abstract: The aromatic profile of a wine is one of the main characteristics appreciated by consumers. Due to climate change, vineyards need to adapt to new conditions, and one of the strategies that might be followed is to develop new white varieties from Monastrell and other cultivars by means of intervarietal crosses, since white varieties are a minority in south-eastern Spain. Such crosses have already been obtained and have been seen to provide quality white wines of high acidity and with a good aromatic composition. To confirm this, a quantitative analysis was carried out during two vintages (2018 and 2019) in order to study and compare the volatile composition of Verdejo (V) wine with the aromatic composition of several wines made from different crosses between Cabernet Sauvignon (C), Syrah (S), Tempranillo (T), and Verdejo (V) with Monastrell (M), by means of headspace SPME-GC-MS analysis. -

Effects on Varietal Aromas During Wine Making: a Review of the Impact of Varietal 1 2 Aromas on the Flavor of Wine
Effects on varietal aromas during wine making: a review of the impact of varietal 1 2 aromas on the flavor of wine. 3 4 5 6 a b c d a 7 Javier Ruiz , Florian Kiene , Ignacio Belda , Daniela Fracassetti , Domingo Marquina , 8 e e e b a* 9 Eva Navascués , Fernando Calderón , Angel Benito , Doris Rauhut , Antonio Santos , 10 Santiago Benitoe* 11 12 13 14 15 a 16 Department of Genetics, Physiology and Microbiology, Biology Faculty, Complutense 17 18 University of Madrid, 28040 Madrid, Spain 19 20 bDepartment of Microbiology and Biochemistry, Hochschule Geisenheim University, 21 22 65366 Geisenheim, Germany 23 24 c 25 Department of Biology, Geology, Physics & Inorganic Chemistry. Unit of Biodiversity 26 27 and Conservation. Rey Juan Carlos University, 28933 Móstoles, Spain 28 29 dDepartment of Food, Environmental and Nutritional Sciences, Università degli Studi di 30 31 Milano, Milan, Italy 32 33 34 eDepartment of Chemistry and Food Technology. Escuela Técnica Superior de Ingeniería 35 36 Agronómica, Alimentaria y de Biosistemas, Polytechnic University of Madrid, Ciudad 37 38 Universitaria S/N, 28040 Madrid, Spain 39 40 41 42 43 *Corresponding authors: 44 45 46 Santiago Benito. Email: [email protected] 47 48 Antonio Santos. Email: [email protected] 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 1 2 3 ABSTRACT 4 5 Although there are many chemical compounds present in wines, only a few of these 6 7 compounds contribute to the sensory perception of wine flavor. This review focuses on 8 9 the knowledge regarding varietal aroma compounds, which are among the compounds 10 11 that are the greatest contributors to the overall aroma. -

Comprehensive Study of Australian Rosé Wines: Characterisation of Chemical and Sensory Profiles
COMPREHENSIVE STUDY OF AUSTRALIAN ROSÉ WINES: CHARACTERISATION OF CHEMICAL AND SENSORY PROFILES Jiaming Wang A thesis submitted for the degree of Doctor of Philosophy School of Agriculture, Food and Wine Faculty of Sciences The University of Adelaide March 2016 Table of Contents Thesis summary i Declaration iv Publications v Panel of supervisors vi Acknowledgements vii Chapter 1 Literature review 1 1.1 Wine aroma 2 1.1.1 Grape-derived aroma compounds 2 1.1.2 Aroma compounds derived from pre-fermentation processes 8 1.1.3 Volatiles derived from fermentation 8 1.1.4 Ageing-related volatiles 12 1.2 Wine aroma analysis 15 1.2.1 The importance and outcome 15 1.2.2 Instrumentation 16 1.2.3 Sample preparation 23 1.2.4 Procedures for analysis of wine aroma 27 1.3 Perspective 35 1.4 Rosé wine 36 1.4.1 Definition and winemaking techniques 36 1.4.2 Aroma in rosé wine 37 Summary of research aims 45 References 47 Chapter 2 Chemical and sensory profiles of rosé wines from Australia 61 Chapter 3 Rosé wine volatile composition and the preferences of Chinese wine professionals 76 Chapter 4 Comprehensive study of volatile compounds in two Australian rosé wines: aroma extract dilution analysis (AEDA) of extracts prepared using solvent-assisted flavor evaporation (SAFE) or headspace solid-phase extraction (HS-SPE) 90 Chapter 5 Concluding remarks and future perspectives 103 5.1 Conclusions 104 5.1.1 Chemical and sensorial profiles of Australian rosé wines 105 5.1.2 Chinese wine professionals’ preferences for rosé wines and links between wine composition and quality 106 5.1.3 AEDA study on rosé wines and strategies to produce a representative extract for GC- O analysis 107 5.2 Future directions 108 List of abbreviations 112 Appendix 115 References 141 Thesis Summary Rosé wine is a versatile and diverse style which is increasing in popularity in Australia and elsewhere, and the development of new markets such as China offers great potential to the Australian wine industry. -

Wine from Cold Hardy Grapes
Making, Blending, and Selling Wines From Cold Hardy Cultivars Stephen Menke Colorado State University-WCRC Desirable Traits in Hybrid Grape Wines Great fruitiness Usually good color Sufficient acid Great taste intensity upon presentation to mouth Good food pairing Good dry or sweet Problem Traits in Hybrid Grape Wines Some strong varietal aromas and tastes Can be too acid Tannins low Prone to structural breakdown of flavor and body Sweeter wines prone to re-fermentation Cool Climate Vitis vinifera Intraspecific Crosses Cool Climate = winter minimum of -50F to -150F, and depends on acclimation Lemberger (red, moderate cold resistance, fruity, good wine quality) Comtessa (red used for white, moderate cold resistance, fruity wine) Siegerrebe (white, fairly cold resistant, very floral wine) Noblessa (white, moderate cold resistance, good wine quality reported) Morio muscat (white, moderate cold, northeast US, very floral and fruity) Madeleine Angevine (white, moderate cold, good wine quality reported) Cool Climate Hybrids/Natives vinifera/American, vinifera/amurensis, Cornell, Minnesota, UC Davis Useful site http://viticulture.hort.iastate.edu/cultivars/cultivars.html Cool Climate = winter minimum of -50F to -150F, and depends on acclimation Reds Baco noir, Chambourcin, Chancellor, Concord, Corot noir, Crimson cabernet, DeChaunac, GR7, Kozma 55, Kozma 525, Landot noir, Leon Millot, Marechal Foch, Noiret, Norton, St. Vincent Cool Climate Hybrids/Natives vinifera/American, vinifera/amurensis, Cornell, Minnesota, UC Davis Cool Climate = winter minimum of -50F to -150F, and depends on acclimation Whites Catawba (rosé), Cayuga white, Chardonel, Delaware, Niagara, Seyval blanc, Traminette, Valvin muscat, Veeblanc, Vidal blanc, Vignoles Cold Climate Hybrids Swenson, Minnesota, Cornell, etc. Cold Climate = winter minimum of -150F to - 300F, depends on acclimation Reds Baltica, Frontenac, Marquette, MN 1200, Sabrevois, St. -

Novel Analysis on Aroma Compounds of Wine, Vinegar and Derived Products
Novel Analysis on Aroma Compounds of Wine, Vinegar and Derived Products Edited by Enrique Durán-Guerrero and Remedios Castro-Mejías Printed Edition of the Special Issue Published in Foods www.mdpi.com/journal/foods Novel Analysis on Aroma Compounds of Wine, Vinegar and Derived Products Novel Analysis on Aroma Compounds of Wine, Vinegar and Derived Products Editors Enrique Dur´an-Guerrero Remedios Castro-Mej´ıas MDPI Basel Beijing Wuhan Barcelona Belgrade Manchester Tokyo Cluj Tianjin • • • • • • • • • Editors Enrique Duran-Guerrero´ Remedios Castro-Mej´ıas University of Cadiz´ University of Cadiz´ Spain Spain Editorial Office MDPI St. Alban-Anlage 66 4052 Basel, Switzerland This is a reprint of articles from the Special Issue published online in the open access journal Foods (ISSN 2304-8158) (available at: https://www.mdpi.com/journal/foods/special issues/Wine Aroma). For citation purposes, cite each article independently as indicated on the article page online and as indicated below: LastName, A.A.; LastName, B.B.; LastName, C.C. Article Title. Journal Name Year, Volume Number, Page Range. ISBN 978-3-0365-0000-0 (Hbk) ISBN 978-3-0365-0000-0 (PDF) © 2021 by the authors. Articles in this book are Open Access and distributed under the Creative Commons Attribution (CC BY) license, which allows users to download, copy and build upon published articles, as long as the author and publisher are properly credited, which ensures maximum dissemination and a wider impact of our publications. The book as a whole is distributed by MDPI under the terms and conditions of the Creative Commons license CC BY-NC-ND.