Chemical Composition of Dietary Fiber and Polyphenols of Wine Grape Pomace Skins and Development of Wine Grape (Cv
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

B. F. Clyde's Cider Mill
B. F. Clyde’s Cider Mill Established 1898 Old Mystic, Connecticut National Mechanical Engineering Site Dedication October 29, 1994 The American Society of Mechanical Engineers required equipment that was operated only once a History of Cider in the U.S. year, farmers found it more convenient to travel consid- Apple cider dates back to the earliest days of erable distances to bring their fruit to a large mill for English settlement in the thirteen colonies. Colonists processing into juice (sweet cider). Surplus apples brought seed from England to plant apple trees. could be sold or bartered to the mill owner who would Later, seedlings and whole trees were transported to produce cider to sell. Farmers returned home and used the colonies by wealthier colonists who established their own method of fermentation to produce cider. large apple orchards. Although apples were a staple In 1881, Mr. Ben- in the meager diet of jamin F. Clyde decided early settlers, the moti- to produce and sell cider vation for raising apple in Mystic, now referred trees was equally for to as Old Mystic. For the the purpose of making first few years, he cider. Cider was easy pressed his apples at lo- to make, stored well, cal mills. Eventually, he and provided a mildly bought a press and in- alcoholic drink for all to stalled it in rented space enjoy. Until approxi- in the corner of a local mately seventy years saw mill. He received ago, cider was what is power for his press from now referred to as “hard the saw mill’s line shaft. -

GRAS Notice (GRN) No. 719, Orange Pomace
GRAS Notice (GRN) No. 719 https://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/default.htm SAFETY EVALUATION DOSSIER SUPPORTING A GENERALLY RECOGNIZED AS SAFE (GRAS) CONCLUSION FOR ORANGE POMACE SUBMITTED BY: PepsiCo, Inc. 700 Anderson Hill Road Purchase, NY 10577 SUBMITTED TO: U.S. Food and Drug Administration Center for Food Safety and Applied Nutrition Office of Food Additive Safety HFS-200 5100 Paint Branch Parkway College Park, MD 20740-3835 CONTACT FOR TECHNICAL OR OTHER INFORMATION: Andrey Nikiforov, Ph.D. Toxicology Regulatory Services, Inc. 154 Hansen Road, Suite 201 Charlottesville, VA 22911 July 3, 2017 Table of Contents Part 1. SIGNED STATEMENTS AND CERTIFICATION ...........................................................1 A. Name and Address of Notifier .............................................................................................1 B. Name of GRAS Substance ...................................................................................................1 C. Intended Use and Consumer Exposure ................................................................................1 D. Basis for GRAS Conclusion ................................................................................................2 E. Availability of Information ..................................................................................................3 Part 2. IDENTITY, METHOD OF MANUFACTURE, SPECIFICATIONS, AND PHYSICAL OR TECHNICAL EFFECT.................................................................................................4 -

Grape Pomace Valorization: a Systematic Review and Meta-Analysis
foods Review Grape Pomace Valorization: A Systematic Review and Meta-Analysis Bojan Antoni´c 1 , Simona Janˇcíková 1 , Dani Dordevi´c 1,2,* and Bohuslava Tremlová 1 1 Department of Plant Origin Foodstuffs Hygiene and Technology, Faculty of Veterinary Hygiene and Ecology, University of Veterinary and Pharmaceutical Sciences, 61242 Brno, Czech Republic; [email protected] (B.A.); [email protected] (S.J.); [email protected] (B.T.) 2 Department of Technology and Organization of Public Catering, South Ural State University, Lenin Prospect 76, 454080 Chelyabinsk, Russia * Correspondence: [email protected] Received: 2 October 2020; Accepted: 5 November 2020; Published: 7 November 2020 Abstract: This systematic review aimed to collect data and analyze the possible use of grape pomace, a winemaking industry byproduct, in the production of fortified foods. The English articles found in Web of Science, Scopus, and Google Scholar, from January 2006 until May 2020, were used for the conduction of overview tables and meta-analysis. The systematic review emphasized the two main issues concerning grape pomace application to other food products: (i) grape pomace contains high amounts of health promoting compounds; and (ii) the use of grape pomace is influencing the waste management. The grape pomace has been used in the fortification of plant origin food, meat, fish, and dairy products, mainly due to higher polyphenols and dietary fiber contents. The fortification was declared as successful in all studied food types. The change of color, caused by polyphenolic compounds, was mainly observed as an adverse effect of the fortification. Higher levels of fortification also caused notable undesirable changes in texture. -

Growing Vitis Vinifera In
Report of research sponsored by the New York State Wine and Grape Foundation hi(T Produced by Communications Services NYS Agricultural Experiment Station Cornell University • Geneva GROWING VITIS VINIFERA GRAPES IN NEW YORK STATE I - Performance of New and Interesting Varieties WRITTEN BY Robert M. Pool 1, Gary E. Howard2, Richard Dunst3, John Dyson4, Thomas Henick-Kling5, Jay Freer 6, Larry Fuller-Perrine 7, Warren Smith8 and Alice Wise 9 1 Professor of Viticulture, Department of Horticultural Sciences, Cornell University, New York State Agricultural Experiment Station, Geneva, NY 2 Research Support Specialist, Department of Horticultural Sciences, Cornell University, New York State Agricultural Experiment Station, Geneva, NY 3 Research Support Specialist, Department of Horticultural Sciences, Cornell University, New York State Agricultural Experiment Station, Fredonia, NY • Veraison Wine Cellars, Millbrook, NY. 'Assistant Professor of Enology, Department of Food Science and Technology, Cornell University, New York State Agricultural Experiment Station, Geneva, NY 6 Administrative Manager, Department of Horticultural Sciences, Cornell University, New York State Agricultural Experiment Station, Geneva, NY 7 Research Support Specialist, Department of Horticultural Sciences, Cornell University, New York State Agricultural Experiment Station, Riverhead, NY 8 Cornell Cooperative Extension, illster County, Highland, NY °Cornell Cooperative Extension, Suffolk County, Riverhead, NY CONTENTS FoREWORD ...........................................................................................i -

Notes on Composting Grape Pomace Fritz Westover Viticulture Research-Extension Associate [email protected]
Notes on Composting Grape Pomace Fritz Westover Viticulture Research-Extension Associate [email protected] Wine producers in the state of Virginia have shown increasing interest in producing compost from wine grape pomace, which can then be applied to vineyard soils as a nutrient rich soil conditioner. The notes below have been compiled to provide a quick reference guide for farm wineries initiating small or large scale composting operations. • pomace is high in N>K>Ca [N-P-K-Ca = 2.0-0.5-2.0-2.0] • pomace is about 8% seeds, 10% stems, 25% skins, 57% pulp • in general 1 ton of harvested grapes produces 100lbs of stems and 160 to 240 lbs of pomace (more simply, 3 tons grapes is about equal to 1 ton of total pomace) • returns ½ to 1/3 of nutrients and OM removed from crop • 1:1 ratio, pomace:manure bedding (straw + manure) provides 2/3 to 100% annual nutrient needs of vineyard • pomace alone composts’ slowly – low pH (3.5 to 3.8) • compost microbes prefer a pH of 6.2 to become active (pH >6 desired) • lime or other feedstocks must be added to the pomace in order to increase pH • pomace has C:N ratio appropriate for composting (1:17 to 1:30) • feedstock added to pomace should also have C:N ratio appropriate for composting (1:20 to 1:30) • high lignin in seeds (17to 35%) limits decomposition in unturned piles • wet piles (>60% moisture) may continue to ferment, produce acetic acid = poor quality (check for off odors in pile or other clues of anaerobic activity) • 1-5 tons per acre annually is considered maintenance application • frequent turning -

Total and Sustainable Valorisation of Olive Pomace Using a Fractionation Approach
applied sciences Article Total and Sustainable Valorisation of Olive Pomace Using a Fractionation Approach Tânia B. Ribeiro 1,2 , Ana L. Oliveira 1, Cristina Costa 2, João Nunes 1, António A. Vicente 3 and Manuela Pintado 1,* 1 CBQF-Centro de Biotecnologia e Química Fina–Laboratório Associado, Escola Superior de Biotecnologia, Universidade Católica Portuguesa, Rua Diogo Botelho 1327, 4169-005 Porto, Portugal; [email protected] or [email protected] (T.B.R.); [email protected] (A.L.O.); [email protected] (J.N.) 2 Centre Bio R&D Unit, Association BLC3-Technology and Innovation Campus, Rua Nossa Senhora da Conceição, 2, Lagares, 3405-155 Oliveira do Hospital, Portugal; [email protected] 3 Centro de Engenharia Biológica, Universidade do Minho, Campus Gualtar, 4710-057 Braga, Portugal; [email protected] * Correspondence: [email protected]; Tel.: +351-22-55-800-44 Received: 14 August 2020; Accepted: 23 September 2020; Published: 28 September 2020 Abstract: Olive pomace management represents a great concern to the olive oil industry. This work focused on the development of a “zero waste” strategy for olive pomace based on a fractionation approach resulting in the obtention of different value-added fractions. The physicochemical composition of edible fractions obtained (liquid and pulp) was analysed. The potential use as a solid biofuel of the non-edible fraction (stones) was evaluated. High amounts of hydroxytyrosol (513.61–625.76 mg/100 g dry weight) were present in the liquid fraction. Pulp fraction was demonstrated to be a good source of fibre (53–59% dry weight) with considerable antioxidant activity both from free and bound phenolics. -

Wine Contamination with Ochratoxins: a Review
beverages Review ReviewWine Contamination with Ochratoxins: A Review Wine Contamination with Ochratoxins: A Review Jessica Gil-Serna 1,* ID , Covadonga Vázquez 1, María Teresa González-Jaén 2 ID and JessicaBelén Gil-Serna Patiño 1 ID1,*, Covadonga Vázquez 1, María Teresa González-Jaén 2 and Belén Patiño 1 1 1DepartmentDepartment of Microbiology of Microbiology III, III,Faculty Faculty of Biolo of Biology,gy, University University Complutense Complutense of Madrid, of Madrid, Jose Antonio NovaisJose 12, Antonio 28040 NovaisMadrid, 12, Spain; 28040 [email protected] Madrid, Spain; (C.V.); [email protected] [email protected] (C.V.); (B.P.) [email protected] (B.P.) 2 2DepartmentDepartment of Genetics, of Genetics, Faculty Faculty of Biology, of Biology, Univer Universitysity Complutense Complutense of Madrid, of Madrid, Jose Jose Antonio Antonio Novais Novais 12, 12, 2804028040 Madrid, Madrid, Spain; Spain; [email protected] [email protected] * *Correspondence:Correspondence: jgilsern [email protected];@ucm.es; Tel.: Tel.: +34-91-394-4969 +34-91-394-4969 Received:Received: 31 31October October 2017; 2017; Accepted: Accepted: 29 29December December 2017; 2017; Published: Published: 29 15January January 2018 2018 Abstract:Abstract: OchratoxinOchratoxin A A (OTA) isis thethe main main mycotoxin mycotoxin occurring occurring inwine. in wine. This This review review article article is focused is focusedon the on distribution the distribution of this of toxin this andtoxin its and producing-fungi its producing-fungi in grape in grape berries, berries, as well as aswell on as the on fate theof fateOTA of duringOTA during winemaking winemaking procedures. procedures. Due to itsDue toxic to its properties, toxic properties, OTA levels OTA in winelevels are in regulated wine arein regulateddifferent in countries; different therefore, countries; it is therefore, necessary toit applyis necessary control andto apply detoxification control methodsand detoxification that are also methodsdiscussed that in are this also revision. -

Wine of Origin Booklet
Version 20101201 TABLE OF CONTENTS Introduction ....................................................................................................................................... 3 Wine and Spirit Board ........................................................................................................................ 3 Composition ....................................................................................................................................... 3 Functions ............................................................................................................................................ 3 Operation ........................................................................................................................................... 4 Wine of Origin Scheme ...................................................................................................................... 6 Importance of Origin .......................................................................................................................... 6 Demarcation of areas of Origin .......................................................................................................... 6 Criteria for the demarcation of areas of Origin ................................................................................. 7 Geographical unit ............................................................................................................................... 8 The role of cultivar in Wine of Origin ................................................................................................ -

Canada-EU Wine and Spirits Agreement
6.2.2004 EN Official Journal of the European Union L 35/3 AGREEMENT between the European Community and Canada on trade in wines and spirit drinks THE EUROPEAN COMMUNITY, hereafter referred to as ‘the Community', and CANADA, hereafter jointly referred to as ‘the Contracting Parties', RECOGNISING that the Contracting Parties desire to establish closer links in the wine and spirits sector, DESIROUS of creating more favourable conditions for the harmonious development of trade in wine and spirit drinks on the basis of equality and mutual benefit, HAVE AGREED AS FOLLOWS: TITLE I — ‘labelling' shall mean any tag, brand, mark, pictorial or other descriptive matter, written, printed, stencilled, marked, embossed or impressed on, or attached to, a INITIAL PROVISIONS container of wine or a spirit drink, Article 1 — ‘WTO Agreement' refers to the Marrakesh Agreement establishing the World Trade Organisation, Objectives 1. The Contracting Parties shall, on the basis of — ‘TRIPs Agreement' refers to the Agreement on trade-related non-discrimination and reciprocity, facilitate and promote aspects of intellectual property rights, which is contained trade in wines and spirit drinks produced in Canada and the in Annex 1C to the WTO Agreement, Community, on the conditions provided for in this Agreement. 2. The Contracting Parties shall take all reasonable measures — ‘1989 Agreement' refers to the Agreement between the to ensure that the obligations laid down in this Agreement are European Economic Community and Canada concerning fulfilled and that the objectives set out in this Agreement are trade and commerce in alcoholic beverages concluded on attained. 28 February 1989. Article 2 2. -

Ethanol Recovery from Solid State Fermented Apple Pomace and Evaluation of Physico-Chemical Characteristics of the Residue
Natural Product Radiance, Vol. 7(2), 2008, pp.127-132 Research Paper Ethanol recovery from solid state fermented apple pomace and evaluation of physico-chemical characteristics of the residue V K Joshi*and A Devrajan Department of Postharvest Technology Dr Y S Parmar University of Horticulture and Forestry Nauni, Solan-173 230, Himachal Pradesh, India *Correspondent author, E-mail: [email protected]; Phone: +91-01792-252410 Received 23 April 2007; Accepted 29 November 2007 handled properly. Thus, there is a strong Abstract In view of the growing demand of ethanol the identification of resources and development need to devise techniques to utilize apple of economical methods for its extraction are very essential. Fermented apple pomace has been pomace in an economical and effective identified as a rich source of ethanol especially for the Himalayan region where apple is grown at way to avoid the environmental pollution large scale. There are various methods of alcohol recovery from solid state fermented apple and to obtain value-added products from pomace (hot water extraction followed by distillation, vacuum distillation, hydraulic pressure and waste materials3-5. direct steam distillation) hence, present study was carried out to standardize an efficient and economical method. The physico-chemical characteristics of dried apple pomace residue after the Use of apple pomace has been recovery of ethanol by different methods were also evaluated for knowing loss of nutrients during made through fermentation into several extraction of ethanol. For present study two types of solid state fermented (SSF) apple pomace, products including citric acid, ethanol, obtained by two treatments (one by Saccharomyces cerevisiae and other by Candida utilis pigment, mushroom substrate, single cell and Kloeckera spp. -
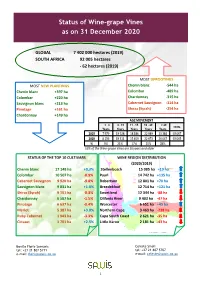
Status of Wine-Grape Vines As on 31 December 2020
Status of Wine-grape Vines as on 31 December 2020 GLOBAL 7 402 000 hectares (2019) SOUTH AFRICA 92 005 hectares - 62 hectares (2019) MOST UPROOTINGS MOST NEW PLANTINGS Chenin blanc -544 ha Chenin blanc +397 ha Colombar -489 ha Colombar +220 ha Chardonnay -315 ha Sauvignon blanc +213 ha Cabernet Sauvignon -314 ha Pinotage +161 ha Shiraz (Syrah) -254 ha Chardonnay +149 ha AGE MOVEMENT < 4 4 - 10 11 - 15 16 - 20 > 20 TOTAL Years Years Years Years Years 2019 7 979 19 528 18 186 22 989 23 384 92 067 2020 8 156 19 311 15 819 22 673 26 047 92 005 % 9% 21% 17% 25% 28% 53% of the Wine-grape Vines are 16 years and older STATUS OF THE TOP 10 CULTIVARS WINE REGION DISTRIBUTION (2020/2019) Chenin blanc 17 148 ha +0,3% Stellenbosch 15 085 ha +19 ha Colombar 10 507 ha -0.9% Paarl 14 742 ha +135 ha Cabernet Sauvignon 9 920 ha -9.8% Robertson 12 801 ha +70 ha Sauvignon blanc 9 831 ha +1.8% Breedekloof 12 714 ha +121 ha Shiraz (Syrah) 9 151 ha -0.3% Swartland 12 344 ha -88 ha Chardonnay 6 587 ha -1.5% Olifants River 9 403 ha -47 ha Pinotage 6 637 ha -0.4% Worcester 6 651 ha +45 ha Merlot 5 387 ha +3.0% Northern Cape 3 463 ha -238 ha Ruby Cabernet 1 943 ha -3.3% Cape South Coast 2 621 ha -35 ha Cinsaut 1 701 ha +2.5% Little Karoo 2 181 ha -43 ha Bonita Floris-Samuels Celeste Small tel: +27 21 807 5711 tel: +27 21 807 5707 e-mail: [email protected] e-mail: [email protected] 1 Statistics i.r.o. -

Concours Mondial De Bruxelles 2012 | Wine Categories Concours Mondial De Bruxelles 2012 | Spirits Categories
Concours Mondial de Bruxelles 2012 | Wine Categories Still Rosé Wines 1.1. Grand Duchy of Luxembourg 2.10.12. Sparkling wines from Hungary 2.10.13. aromatic varieties 4.2. Dry Rosé Wines <4 gr/l of residual sugar 1.1.1. Mexico 2.10.14. Rosé Wines >4 gr/l of residual sugar 1.1.2. New Zealand 2.10.15. White sparkling wines from aromatic varieties 4.2.1. Czech Republic white wine <10gl sugar 2.10.16. Rosé sparkling wines from aromatic varieties 4.2.2. Organic rosé Wine 1.6. Czech Republic white wine > 10g/l sugar 2.10.17. Slovakia 2.10.18. Organic Sparkling Wine 4.6. Organic rosé wine <10€ 1.6.1. Slovenia 2.10.19. Organic rosé wine >10€ 1.6.2. Switzerland Valais 2.10.20. Organic sparkling wine <10€ 4.6.1. Switzerland other regions 2.10.21. Organic sparkling wine >10€ 4.6.2. White wines from Turkey 2.10.22. non-aromatic varieties 2.1. USA 2.10.23. Other Sparkling Wines 4.10. Dry white wine <4 gr/l residual sugar 2.1.1. Red Wines 3.1. Sekt 4.10.1. Semi-dry wine <4,1 - 12> gr/l residual sugar 2.1.2. Crémant from Luxembourg 4.10.2. Sweet wines <12,1 - 50> gr/l residual sugar 2.1.3. Red wines <4 gr/l residual sugar 3.1.1. Other countries 4.10.3. Sweet wines > 50 gr/l residual sugar 2.1.4. Red wines >4 gr/l residual sugar 3.1.2.