14 Implantation of Autologous Bone Marrow
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

15-1040-Junu Oh-Neuronal.Key
Neuronal Control of the Bladder Seung-June Oh, MD Department of urology, Seoul National University Hospital Seoul National University College of Medicine Contents Relevant end organs and nervous system Reflex pathways Implication in the sacral neuromodulation Urinary bladder ! body: detrusor ! trigone and bladder neck Urethral sphincters B Preprostatic S Smooth M. Sphincter Passive Prostatic S Skeletal M. Sphincter P Prostatic SS P-M Striated Sphincter Membraneous SS Periurethral Striated M. Pubococcygeous Spinal cord ! S2–S4 spinal cord ! primary parasympathetic micturition center ! bladder and distal urethral sphincter ! T11-L2 spinal cord ! sympathetic outflow ! bladder and proximal urethral sphincter Peripheral innervation ! The lower urinary tract is innervated by 3 principal sets of peripheral nerves: ! parasympathetic -pelvic n. ! sympathetic-hypogastric n. ! somatic nervous systems –pudendal n. ! Parasympathetic and sympathetic nervous systems form pelvic plexus at the lateral side of the rectum before reaching bladder and sphincter Sympathetic & parasympathetic systems ! Sympathetic pathways ! originate from the T11-L2 (sympathetic nucleus; intermediolateral column of gray matter) ! inhibiting the bladder body and excite the bladder base and proximal urethral sphincter ! Parasympathetic nerves ! emerge from the S2-4 (parasympathetic nucleus; intermediolateral column of gray matter) ! exciting the bladder and relax the urethra Sacral somatic system !emerge from the S2-4 (Onuf’s nucleus; ventral horn) !form pudendal nerve, providing -

Clinical and Functional Anatomy of the Urethral Sphincter
Review Article International Neurourology Journal Int Neurourol J 2012;16:102-106 http://dx.doi.org/10.5213/inj.2012.16.3.102 pISSN 2093-4777 · eISSN 2093-6931 INJ Clinical and Functional Anatomy of the Urethral Sphincter Junyang Jung, Hyo Kwang Ahn, Youngbuhm Huh Department of Anatomy, Kyung Hee University School of Medicine, Seoul, Korea Continence and micturition involve urethral closure. Especially, insufficient strength of the pelvic floor muscles including the urethral sphincter muscles causes urinary incontinence (UI). Thus, it is most important to understand the main mechanism causing UI and the relationship of UI with the urethral sphincter. Functionally and anatomically, the urethral sphincter is made up of the internal and the external sphincter. We highlight the basic and clinical anatomy of the internal and the external sphinc- ter and their clinical meaning. Understanding these relationships may provide a novel view in identifying the main mechanism causing UI and surgical techniques for UI. Keywords: Urethral sphincters; Pudendal nerve; Autonomic nervous system; Urinary incontinence; Urination INTRODUCTION tomical damage to the ligaments, facial support, and pelvic floor musculature, including the levator ani [8]. The pudendal nerve The urethral sphincter is crucial for the maintenance of urinary innervating the EUS is susceptible to injury during vaginal birth continence [1,2]. The urethral sphincter refers to one of the fol- because it travels between the sacrospinous and sacrotuberous lowing muscles [3]: 1) the internal urethral sphincter (IUS), ligaments [9]. In this article, we discuss the basic and clinical which consists of smooth muscle and is continuous with the anatomy of the urethral sphincter and the relationship between detrusor muscle and under involuntary control, and 2) the ex- the urethral sphincter and UI. -
Urinary System
OUTLINE 27.1 General Structure and Functions of the Urinary System 818 27.2 Kidneys 820 27 27.2a Gross and Sectional Anatomy of the Kidney 820 27.2b Blood Supply to the Kidney 821 27.2c Nephrons 824 27.2d How Tubular Fluid Becomes Urine 828 27.2e Juxtaglomerular Apparatus 828 Urinary 27.2f Innervation of the Kidney 828 27.3 Urinary Tract 829 27.3a Ureters 829 27.3b Urinary Bladder 830 System 27.3c Urethra 833 27.4 Aging and the Urinary System 834 27.5 Development of the Urinary System 835 27.5a Kidney and Ureter Development 835 27.5b Urinary Bladder and Urethra Development 835 MODULE 13: URINARY SYSTEM mck78097_ch27_817-841.indd 817 2/25/11 2:24 PM 818 Chapter Twenty-Seven Urinary System n the course of carrying out their specific functions, the cells Besides removing waste products from the bloodstream, the uri- I of all body systems produce waste products, and these waste nary system performs many other functions, including the following: products end up in the bloodstream. In this case, the bloodstream is ■ Storage of urine. Urine is produced continuously, but analogous to a river that supplies drinking water to a nearby town. it would be quite inconvenient if we were constantly The river water may become polluted with sediment, animal waste, excreting urine. The urinary bladder is an expandable, and motorboat fuel—but the town has a water treatment plant that muscular sac that can store as much as 1 liter of urine. removes these waste products and makes the water safe to drink. -

The Distal Convoluted Tubule and Collecting Duct
Chapter 23 *Lecture PowerPoint The Urinary System *See separate FlexArt PowerPoint slides for all figures and tables preinserted into PowerPoint without notes. Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Introduction • Urinary system rids the body of waste products. • The urinary system is closely associated with the reproductive system – Shared embryonic development and adult anatomical relationship – Collectively called the urogenital (UG) system 23-2 Functions of the Urinary System • Expected Learning Outcomes – Name and locate the organs of the urinary system. – List several functions of the kidneys in addition to urine formation. – Name the major nitrogenous wastes and identify their sources. – Define excretion and identify the systems that excrete wastes. 23-3 Functions of the Urinary System Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Diaphragm 11th and 12th ribs Adrenal gland Renal artery Renal vein Kidney Vertebra L2 Aorta Inferior vena cava Ureter Urinary bladder Urethra Figure 23.1a,b (a) Anterior view (b) Posterior view • Urinary system consists of six organs: two kidneys, two ureters, urinary bladder, and urethra 23-4 Functions of the Kidneys • Filters blood plasma, separates waste from useful chemicals, returns useful substances to blood, eliminates wastes • Regulate blood volume and pressure by eliminating or conserving water • Regulate the osmolarity of the body fluids by controlling the relative amounts of water and solutes -

Kidney Physiology, Part 1 Objectives
4/24/2020 Ch. 10: Kidney Physiology, part 1 Objectives: 1. Understand renal functions. 2. Review anatomy of the urinary system & kidneys. 3. Understand blood flow to kidneys. 4. Anatomy & physiology of the nephron. 5. Regulation of nephron filtration. 6. Kidney disorders. 1. Functions of Urinary System Regulates: 1. Blood volume - by filtering blood, excreting or reabsorbing water from body as needed (influenced by hormones ADH, ANP, & Aldosterone 2. Blood pressure – by regulating blood volume. 3. Blood osmolarity – by controlling reabsorption/excretion of salts (Na+, Cl-, K+, Ca+2). 4. Blood pH – by controlling reabsorption/excretion of H+ & HCO3- in urine. 5. Endocrine functions: >Calcitrol = increases Ca+2 absorbed from proximal convol. tubule >Erythropoietin = stimulates RBC production >Renin = secreted by JGA causes 1 4/24/2020 Urinary Facts: ─ Kidneys CAN filter 5.5L/40min OR 180 L/day! ─ 99% of filtrate is automatically reabsorbed,, regardless of hydration state ─ 1% might/might not be reabsorbed – depends on hormones. ─ Avg urine output (pee) = 0.7 – 2.0 L/day (30 – 80 ml/hr) • Oliguria = smaller than normal urine output • Polyuria = higher than normal urine output • Anuria = no urine production (BAD) ─ Obligatory water loss = 400 ml (must pee out to rid body of wastes) ─ “osmolality” = osmoles (Osm) of solute per kg of solvent (Osm/kg) ─ “osmolarity” = osmoles (Osm) of solute per liter of solution (Osm/L) More accurate for understanding osmotic effects than mass of solute in solution Different kinds of solute can have different sizes Some solutions may have multiple kinds of solute 2. REVIEW anatomy of Urinary System Kidneys = paired organs, posterior abdominal cavity - 8 – 12 renal lobes per kidney - lobes contain millions of nephrons - adrenal glands on top of kidneys. -

Bladder Dysfunction in Multiple Sclerosis
A RESOURCE FOR HEALTHCARE PROFESSIONALS BLADDER DYSFUNCTION IN MULTIPLE SCLEROSIS Rebecca Lavelle, MD Nancy J. Holland, EdD, RN Nancy C. Reitman, MA, RN Effective diagnosis and management of bladder dysfunction makes it possible for people with multiple sclerosis (MS) to continue their recreational and work activities with comfort, dignity and confidence. It is important to note that: • Bladder dysfunction occurs in approximately 80% of people living with MS, in people with minimal symptoms and those with major impairments. • Bladder symptoms, including frequency, urgency and incontinence, may negatively impact social and vocational activities. • Bladder symptoms are often mismanaged, precipitating such problems as acute urinary retention, damage to the detrusor (primary bladder muscle) and urinary tract infections (UTIs). NORMAL BLADDER FUNCTION The bladder wall consists of three main layers: the mucosa, submucosa, and detrusor muscle. The detrusor, a thick layer of smooth muscle, expands to store urine and contracts to expel urine. Storage and emptying of the bladder are regulated by the internal and external urethral sphincters. Sphincters are normally in a closed position, needing stimulation to open. Continence depends on sphincter–detrusor coordination. When approximately 250 to 300 cc of urine fill the bladder, the internal pressure activates stretch receptors in the bladder wall. The stretch receptors signal the nervous system, small contractile waves occur in the detrusor muscle, and the internal urethral sphincter automatically relaxes. The external sphincter is consciously tightened and the urge to urinate becomes apparent. Voluntary voiding occurs when two actions occur simultaneously: the detrusor muscle contracts to expel the urine and the external sphincter relaxes and opens to allow the urine to pass freely into the urethra and out of the body. -

Esfincterotomia Uretral Por Via Endoscópica Em Gato
Arq. Bras. Med. Vet. Zootec., v.63, n.5, p.1093-1098, 2011 Surgical procedures used for post-traumatic neurogenic bladder in a cat: report case [Procedimentos cirúrgicos utilizados no tratamento da bexiga neurogênica em um gato: relato de caso] P.T. Pavan1, V.J.V. Rossetto1*, S.C. Rahal1, L.C. Vulcano1, J.C.S. Trindade Filho2 1Faculdade de Medicina Veterinária - UNESP - Botucatu, SP 2Faculdade de Medicina - UNESP - Botucatu, SP ABSTRACT A 1-year-old castrated crossbred male cat was referred to the Veterinary Teaching Hospital for evaluation of urinary retention associated with a subluxation at T12-T13 caused by a car accident. Urethral sphincter denervation by transection of hypogastric and pudendal nerves was performed to allow bladder emptying, but after three months post operation the cat had a urinary retention recurrence. Endoscopic urethral sphincterotomy was done resulting in urinary incontinence for four months. Keywords: cat, urinary incontinence, urethral denervation, urethral sphincterotomy RESUMO Um gato de um ano de idade, macho, castrado, sem raça definida, foi encaminhado ao Hospital Veterinário Escola para avaliação de retenção urinária associada à subluxação nas vértebras T12-T13, que foi causada por um acidente automobilístico. Realizou-se a denervação do esfíncter uretral, por transecção dos nervos pudendo e hipogástrico, para permitir o esvaziamento da bexiga, porém três meses após a cirurgia inicial o animal apresentou recorrência da retenção urinária. Esfincterotomia endoscópica uretral foi então realizada, resultando em incontinência urinária por quatro meses. Palavras-chave: gato, incontinência urinária, denervação uretral, esfincterotomia uretral INTRODUCTION (Lorenz and Kornegay, 2004; Yoshimura and Chancellor, 2004; Lees, 2005; De Groat, 2006; The micturition is a conscious act of disposing Dewey, 2008). -

Anourethral Reflex. Description of a Reflex and Its Clinical Significance: Preliminary Study
Paraplegia 30 (1992) 210-213 © 1992 International Medical Society of Paraplegia Anourethral reflex. Description of a reflex and its clinical significance: preliminary study A Shafik MD Professor of Surgery, Faculty of Medicine, Cairo University, Cairo, Egypt. A new reflex which I call the 'ano-urethral reflex' is described and its clinical significance discussed. It was studied in 16 volunteers with normal vesical and rectal functions. A concentric needle electrode was introduced into the external anal sphincter (stimulating), and another one into the external urethral sphincter (recording). The reflex response was recorded by EMG apparatus. The external urethral sphincter basal activity was increased with stimulation of the external anal sphincter. This reflex response was absent when the external anal sphincter was anesthetised. The average latency of the reflex was 129.5 ms. Reflex urethral sphincter contraction at defecation guards against involuntary micturition which may result from rectal distension and levator contraction. The anourethral reflex can be a valuable diagnostic tool in routine investigations of patients with urological and proctological complaints. Key words: anal canal; anal sphincters; urethral sphincters; defecation; micturition; reflexes; anourethral reflexes. Introduction External anal and urethral sphincters act Mechanisms of defecation, micturition and as the continent muscles for the rectum and continence are regulated by reflex actions. the urinary bladder respectively. During our Study of the latency, amplitude and dura electrophysiologic study of the relation of tion of these reflexes could play a role in the the 2 continent muscles to each other, it was diagnosis of pathological anorectal and urin found that the external urethral sphincter ary conditions. -
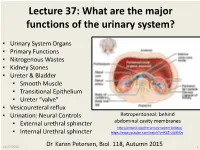
Lecture 37: What Are the Major Functions of the Urinary System?
Lecture 37: What are the major functions of the urinary system? • Urinary System Organs • Primary Functions • Nitrogenous Wastes • Kidney Stones • Ureter & Bladder • Smooth Muscle • Transitional Epithelium • Ureter “valve” • Vesicoureteral reflux • Urination: Neural Controls Retroperitoneal: behind • External urethral sphincter abdominal cavity membranes http://antranik.org/the-urinary-system-kidneys/ • Internal Urethral sphincter https://www.youtube.com/watch?v=K3Z -Lt58H0s 11/27/2016 Dr. Karen Petersen, Biol. 118, Autumn 2015 1 What are the major parts of the urinary system? 11/27/2016 http://classes.midlandstech.edu/carterp/Courses/bio211/chap25/chap25.htm 2 What are the major functions of the kidneys? 1. Remove toxic nitrogenous (N) wastes 2. Regulate blood pressure & water balance 3. Regulate plasma pH 4. Regulate numerous minerals, Na+ , K+, Ca+2 5. Produce EPO & TPO - to regulate blood cell production Compare these nitrogenous wastes from amino acids & nucleic acids: Water soluble Water soluble Largest, not water soluble Protein catabolism Made in liver Nucleic acid catabolism Most toxic Most common Least toxic What causes different types of kidney stones? Calcium Oxalates • Genetic: Metabolic Problem • Oxalates (common) • Uric acid • Cystine (rare) • Geography: • Southern states in U.S. • Warm, dry climates Uric acid • Dehydration • Diet: • High sodium, animal protein…. • May not matter, if not genetic • Medications: • Excess Vitamin A & D Calcium phosphates Calcium in urine • Some antibiotics http://kidneystones.uchicago.edu/files/PIE-CHART-OF-STONES.jpg -

The Urinary System Consists of Kidneys, Ureters, Urinary Bladder
Anatomy Lecture Notes Chapter 23 the urinary system consists of kidneys, ureters, urinary bladder, and urethra the function of the kidneys is NOT "to make urine" the kidneys: 1) regulate water balance 2) regular ECF electrolyte levels (Na, K, Ca) 3) eliminate some metabolic wastes urine is a by-product of these functions A. kidneys 1. located against posterior abdominal wall (retroperitoneal) T11 or T12 to L3 right kidney lower than left kidney 2. surrounded by a. pararenal fat (posterior only) b. renal fascia c. adipose capsule - perirenal fat d. renal capsule - dense c.t. covering surface of kidney e. parietal peritoneum Strong/Fall 2008 Anatomy Lecture Notes Chapter 23 3. layers a. cortex - contains renal corpuscles and extends inwards as renal columns b. medulla - consists of renal pyramids which consist mostly of collecting ducts papilla - apex of renal pyramid; where collecting ducts drain into calyx 4. cavities and associated structures a. renal sinus - space in medial part of kidney; contains renal pelvis b. renal pelvis - expanded superior part of ureter minor calyx collects urine from one renal papilla major calyx formed by junction of 2 or more minor calyces renal pelvis formed by junction of all major calyces Strong/Fall 2008 Anatomy Lecture Notes Chapter 23 5. renal hilum - medial indentation; where ureter leaves kidney 6. blood flow through the kidney - renal fraction = 20% of cardiac output aorta renal artery segmental arteries lobar arteries interlobar arteries arcuate arteries cortical radiate (interlobular) arteries afferent arterioles glomerular capillaries (glomerulus) efferent arteriole peritubular capillaries and vasa recta cortical radiate (interlobular) veins arcuate veins interlobar veins renal vein inferior vena cava Strong/Fall 2008 Anatomy Lecture Notes Chapter 23 7. -
Anatomy & Physiology
Martini’s Essentials of Anatomy and Physiology 8e brings a legacy of superb illustration and text–art integration with a suite of new digital tools The New Eighth edition takes the most popular features from the book and builds them out into effective interactives within the eText and Mastering A&P™ MARTINI / BARTHOLOMEW ESSENTIALS OF Anatomy & Physiology EIGHTH EDITION A01_MART3804_08_SE_WALK.indd 1 07/11/2018 09:28 Spotlight Figures Visually Summarize Difficult Physiological Processes, Making Them Easier to Understand These highly visual one- and two-page presentations of tough topics provide a bridge between readings and related figures and photos to communicate information in a student-friendly, visually effective format. Figure 8-9 SPOTLIGHT PROPAGATION OF AN ACTION POTENTIAL a a bb ContinuousContinuous Propagation Propagation AxonAxon hillock hillock SaltatorySaltatory Propagation Propagation 21 21 3 3 alongalong an an Unmyelinated Unmyelinated Axon Axon InitialInitial segment segment alongalong a a Myelinated Myelinated Axon Axon InIn an an unmyelinated unmyelinated axon, axon, an an action action potential potential moves moves BecauseBecause myelin myelin limits limits the the movement movement of of ions ions 11 22 33 alongalong by by continuous continuous propagation. propagation. The The action action acrossacross the the axon axon membrane, membrane, the the action action potentialpotential spreads spreads by by depolarizing depolarizing the the adjacent adjacent region region potentialpotential must must “jump” “jump” from from node node to to node node ofof the the axon axon membrane. membrane. This This process process continues continues to to duringduring propagation. propagation. This This results results in in much much spreadspread as as a achain chain reaction reaction down down the the axon. -
Changes in Urethral Sphincter Size Following Rehabilitation in Older Women with Stress Urinary Incontinence
Changes in urethral sphincter size following rehabilitation in older women with stress urinary incontinence Stéphanie J. Madill, Stéphanie Pontbriand-Drolet, An Tang, Chantale Dumoulin First Online: 25 September 2014 Abstract Introduction and hypothesis The purpose of this study was to evaluate the effects of a pelvic floor muscle (PFM) rehabilitation program on the striated urethral sphincter in women over 60 years with stress urinary incontinence (SUI). We hypothesized that the PFM rehabilitation program would also exercise the striated urethral sphincter and that this would be demonstrated by hypertrophy of the sphincter on magnetic resonance imaging (MRI). Methods Women with at least weekly episodes of SUI were recruited. Participants were evaluated before and after a 12-week group PFM rehabilitation intervention with T2-weighted fast-spin-echo MRI sequences recorded in the axial plane at rest to assess urethral sphincter size. Data on SUI symptoms and their bother were also collected. No control group was included. Results Seventeen women participated in the study. The striated urethral sphincter increased significantly in thickness (21 %, p < 0.001), cross-sectional area (20 %, p = 0.003), and volume (12 %, p = 0.003) following the intervention. The reported number of incontinence episodes and their bother also decreased significantly. Conclusions This study appears to demonstrate that PFM training for SUI also trains the striated urethral sphincter and that improvement in incontinence signs and symptoms is associated with sphincter hypertrophy in older women with SUI. These findings support previous ultrasound (US) data showing an increase in urethral cross-sectional area following PFM training and extend the previous findings by more specifically assessing the area of hypertrophy and by demonstrating that older women present the same changes as younger women when assessed using MRI data.