Welding, Brazing, and Thermal Cutting
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fundamentals of Joining Processes
Outline ME3072 – MANUFACTURING ENGINEERING II BSc Eng (Hons) in Mechanical Engineering • Introduction to Welding Semester - 4 • Fusion-Welding Processes • Solid-State Welding Processes Fundamentals of Joining • Metallurgy of Welding Processes • Weld Quality • Brazing & Soldering Prepared By : R.K.P.S Ranaweera BSc (Hons) MSc Lecturer - Department of Mechanical Engineering University of Moratuwa 2 (for educational purpose only) Joining Processes Classification of Joining Processes 3 4 1 Introduction to Welding • Attention must be given to the cleanliness of the metal surfaces prior to welding and to possible • Is a process by which two materials, usually metals oxidation or contamination during welding process. are permanently joined together by coalescence, which is induced by a combination of temperature, • Production of high quality weld requires: pressure and metallurgical conditions. Source of satisfactory heat and/or pressure Means of protecting or cleaning the metal • Is extensively used in fabrication as an alternative Caution to avoid harmful metallurgical effects method for casting or forging and as a replacement for bolted and riveted joints. Also used as a repair • Advantages of welding over other joints: medium to reunite metals. Lighter in weight and has a great strength • Types of Welding: High corrosion resistance Fusion welding Fluid tight for tanks and vessels Solid-state (forge) welding Can be altered easily (flexibility) and economically 5 6 • Weldability has been defined as the capacity of • Steps in executing welding: metal to be welded under the fabrication conditions Identification of welds, calculation of weld area by stress imposed into a specific, suitably designed structure analysis, preparation of drawings & to perform satisfactorily in the intended service. -

General Disclaimer One Or More of the Following Statements May Affect This Document
General Disclaimer One or more of the Following Statements may affect this Document This document has been reproduced from the best copy furnished by the organizational source. It is being released in the interest of making available as much information as possible. This document may contain data, which exceeds the sheet parameters. It was furnished in this condition by the organizational source and is the best copy available. This document may contain tone-on-tone or color graphs, charts and/or pictures, which have been reproduced in black and white. This document is paginated as submitted by the original source. Portions of this document are not fully legible due to the historical nature of some of the material. However, it is the best reproduction available from the original submission. Produced by the NASA Center for Aerospace Information (CASI) MASSACHUSETTS INSTITUTE OF TEC'.-INOLOGY DEPARTMENT OF OCEAN ENGINEERING SEP 83 CAMBRIDGE. MASS. 02139 RECEIVED FAVUV wrw STI DEPI- , FINAL REPORT "Wo Under Contract No. NASW-3740 (M.I.T. OSP #93589) ON FEASIBILITY OF REMOTELY MANIPULATED WELDING IN SPACE -A STEP IN THE DEVELOPMENT OF NOVEL JOINING TECHNOLOGIES- Submitted to Office of Space Science and Applications Innovative Utilization of the Space Station Program Code E NASA Headquarters Washington, D.C. 20546 September 1983 by Koichi Masubuchi John E. Agapakis Andrew DeBiccari Christopher von Alt (NASA-CR-1754371 ZEASIbILITY CF RZ,1JTL": Y `84-20857 MANIPJLATED WELLINu iN SPAI.E. A STEP IN THE Uc.Y1;LuPdENT OF NUVLL Ju1NING Tkk ;HNuLUGIES Final Peport (c;dssachu6etts Irist. or Tccli.) U11CIds ibJ p HC Al2/Mk AJ 1 CSCL 1jI G:i/.i7 OOb47 i i rACKNOWLEDGEMENT The authors wish to acknowledge the assistance provided by M.I.T. -

Welding Technology a Suncam Continuing Education Course
033.pdf Welding Technology A SunCam Continuing Education Course Welding Technology By Roger Cantrell www.SunCam.com Page 1 of 35 033.pdf Welding Technology A SunCam Continuing Education Course Learning Objectives This course introduces the student to the concept of developing procedures for welding and brazing. Welding and brazing variables are introduced and some example concepts for applying each variable are highlighted to pique the student’s interest and perhaps lead to further study. Upon completion of this course, the student should be able to: • Understand the concept of creating a welding/brazing procedure • Identify several commonly used welding/brazing processes • Identify the more common welding/brazing variables • Appreciate some of the considerations for applying each variable 1.0 INTRODUCTION This course highlights the basic concepts of developing a welding or brazing procedure specification (WPS/BPS). There are a number of ways to approach this subject such as by process, base material, etc. It will be convenient to organize our thoughts in the format of ASME Section IX. The various factors that might influence weld quality are identified in ASME Section IX as "Welding Variables". "Brazing Variables" are treated in a separate part of Section IX in a manner similar to welding variables. The listing of variables for welding procedures can be found in ASME Section IX, Tables QW-252 through QW-265 (a table for each process). The layout of each table is similar to Figure No. 1. www.SunCam.com Page 2 of 35 033.pdf Welding Technology A SunCam Continuing Education Course Process Variable Variation (Description) Essential Supplementary Essential Nonessential Joint Backing X Root Spacing X Base P Number X Metal G Number X Filler F Number X Metal A Number X Continued in this fashion until all relevant variables for the subject process are listed. -

Mechanical Testing and Evaluation of High-Speed and Low
MECHANICAL TESTING AND EVALUATION OF HIGH-SPEED AND LOW- SPEED FRICTION STIR WELDS A Thesis by Nitin Banwasi Bachelor of Engineering, Bangalore University, Bangalore, India 2000 Submitted to the College of Engineering and the faculty of the Graduate School of Wichita State University in partial fulfillment of the requirements for the degree of Master of Science Fall 2005 EXPERIMENTAL TESTING AND EVALUATION OF HIGH-SPEED AND LOW- SPEED FRICTION STIR WELDS I have examined the final copy of this thesis for form and content and recommend that it be accepted in partial fulfillment of the requirements for the degree of Master of Science, with a major in Mechanical Engineering. George E. Talia, Committee Chair We have read this thesis and recommended its acceptance: Dr. Hamid M. Lankarani, Department Chair, Committee Member Dr. Krishna K. Krishnan, Committee Member ii DEDICATION To My Parents iii ACKNOWLEDGEMENTS I am grateful to all that are part of my efforts during my work both academically and personally. I am thankful to my committee chair, Dr.George E.Talia, for being not only supportive in my endeavors but also patient and informative. I appreciate the involvement of both Dr. Hamid M. Lankarani and Dr. Krishna K. Krishnan for their involvement in its fulfillment. I also want to remember fellow student’s help and suggestions in making it possible with gratitude. iv ABSTRACT The potential of the Friction Stir Welding (FSW) process is easily observed in the creation of defect free welds in almost all of the Aluminum alloys. The success and applicability of the process, however, will depend on the performance of the welds compared to other joining processes. -

The Right Tool for Every Job...Big Or Small
The right tool for every job.... big or small TRANSMIG MULTI-PROCESS WELDING INVERTERS The CIGWELD complete range of TRANSMIG multi-process MIG, Stick & TIG welding inverters, come loaded with features sure to satisfy any trade professional in any industry regardless of the welding application. 1300 654 674 I www.cigweld.com.au Sponsors Index Shindaiwa Structural Steel Standards 2 http://www.shindaiwa.com.au/ South Pacific Welding Group Pressure Equipment 6 http://www.spwgroup.com.au/home.asp Standards Smenco http://www.smenco.com.au Thermadyne – Transmig 9 Thermadyne - Cigweld Range www.thermadyne.com.au SafeTac Company Bio – Boston 11 http://www.safetac.com.au Engineering Bureau Veritas http://www.bureauveritas.com.au Smenco – Mining Spec 13 Southern Cross Industrial Welder Supplies http://www.scis.com.au 1300 Apprentice 15 Technoweld http://www.technoweld.com.au MSA - Australian Government 16 Hardface Technologys Skills Connect http://www.hardface.com.au 3834 Weld Management Letter to the Editor 18 [email protected] Welding Duplex – Lincoln 19 Cover Page Electric CIGWELD have released a complete family of six Transmig 3‐in‐1 MIG, STICK and TIG welding inverters to Progress Update 20 the market, ranging from 175 Amps right up to 550 Amps. In November 2011, the Transmig 200i and Transmig 250i single phase portable Multi‐Process Inverters with power factor correction (PFC) hit the market and created quite a stir, and now in early 2012 CIGWELD have realised the 3 phase versions to complete the Transmig inverter range. AWI operates this service for members. Information and comments in AWI publications are the opinions of specific individuals and companies, and may not reflect the position of AWI or its Directors. -

Welding Operations
WELDING OPERATIONS Date Initiated: February 1, 1993 Dates Modified / Updated: September 15, 1993 October 16, 1998 PROCESS DESCRIPTION: Many industrial and manufacturing facilities regularly use a variety of welding processes and materials. The processes include; - Gas Metal Arc Welding (GMAW) - a. k. a. Metal Inert Gas Welding (MIG), - Gas Tungsten Arc Welding (GTAW) - a. k. a. Tungsten Inert Gas Welding (TIG), - Shielded Metal Arc Welding (SMAW) - a. k. a. Manual Metal Arc Welding (MMA), - Flux Core Arc Welding (FCAW), - Submerged Arc Welding (SAW), - Arc Spot Welding, - Electrogas Welding, - Electrostag Welding, - Brazing, - Thermal Cutting, - Resistance Welding, - Plasma Arc Welding, - Electron Beam Welding, - Laser Beam Welding The majority of the common welding processes can be classified as either gas metal arc welding (GMAW) or shielded metal arc welding (SMAW). GMAW generally uses an electrical current to melt and apply a filler metal under a blanket of inert gas. SMAW traditionally uses an electrical current to melt specially coated electrodes which form a protective flux over the weld during application. Both processes use electrodes, filler metals, wire, coatings, and/or gases that may contain and emit several listed substances including NOx, CO, cadmium, cobalt, copper, chromium, manganese, nickel, lead, zinc, and fluorides. Welding operations release fumes and particulates with diameters of 0.001 to 100 microns. Previous studies of welding emissions have been primarily focused on worker exposure and safety. Many technical difficulties have been identified regarding proper sampling and analytical procedures due, in part, to the wide variety of processes, welding materials, and field conditions. The majority of existing test data which can be used to quantify welding emissions is based on studies performed by the American Welding Society (AWS). -

Guidelines for the Welded Fabrication of Nickel-Containing Stainless Steels for Corrosion Resistant Services
NiDl Nickel Development Institute Guidelines for the welded fabrication of nickel-containing stainless steels for corrosion resistant services A Nickel Development Institute Reference Book, Series No 11 007 Table of Contents Introduction ........................................................................................................ i PART I – For the welder ...................................................................................... 1 Physical properties of austenitic steels .......................................................... 2 Factors affecting corrosion resistance of stainless steel welds ....................... 2 Full penetration welds .............................................................................. 2 Seal welding crevices .............................................................................. 2 Embedded iron ........................................................................................ 2 Avoid surface oxides from welding ........................................................... 3 Other welding related defects ................................................................... 3 Welding qualifications ................................................................................... 3 Welder training ............................................................................................. 4 Preparation for welding ................................................................................. 4 Cutting and joint preparation ................................................................... -

Magnetically Impelled Arc Butt (MIAB) Welding of Chrome Plated Steel
MAGNETICALLY IMPELLED ARC BUTT (MIAB) WELDING OF CHROMIUM- PLATED STEEL TUBULAR COMPONENTS UTILIZING ARC VOLTAGE MONITORING TECHNIQUES DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By David H. Phillips, M.S.W.E ***** The Ohio State University 2008 Dissertation Committee: Professor Charley Albright, Advisor Approved by Professor Dave Dickinson _________________________________ Professor John Lippold Advisor Welding Engineering Graduate Program ABSTRACT Magnetically Impelled Arc Butt (MIAB) welding is a forge welding technique which generates uniform heating at the joint through rapid rotation of an arc. This rotation results from forces imposed on the arc by an external magnetic field. MIAB welding is used extensively in Europe, but seldom utilized in the United States. The MIAB equipment is robust and relatively simple in design, and requires low upset pressures compared to processes like Friction welding. In the automotive industry, tubular construction offers many advantages due to the rigidity, light weight, and materials savings that tubes provide. In the case of automotive suspension components, tubes may be chromium-plated on the ID to reduce the erosive effects of a special damping fluid. Welding these tubes using the MIAB welding process offers unique technical challenges, but with potential for significant cost reduction vs. other welding options such as Friction welding. Based on published literature, this research project represented the first attempt to MIAB weld chromium-plated steel tubes, and to utilize voltage monitoring techniques to assess weld quality. ii Optical and SEM microscopy, tensile testing, and an ID bend test technique were all used to assess the integrity of the MIAB weldments. -

Gas-Shielded Arc Welding TIG Welding
Gas-Shielded Arc Welding Gas-shielded welding can be divided into the tungsten gas-shielded welding and the metal gas-shielded welding processes. The tungsten gas-shielded welding covers the processes − Tungsten plasma arc welding (PAW) − Inert-gas tungsten-arc welding (TIG), whereby TIG welding is the most widely used fusion welding process for aluminium. The plasma welding consists only of the plasma-arc welding process which works with a transferred arc. The metal shielded-gas welding is limited to the metal inert-gas welding process operating with an inert gas as shield, as well as a process combination with plasma welding (plasma metal shielded-gas welding - PMIG). The abbreviations used are: GAW Gas-shielded arc welding GMGMMA Gas-mixture shielded metal-arc welding GTAW Gas-shielded tungsten arc welding (MAGM) GMAW Gas-shielded metal arc welding MAGC CO2-shielded metal-arc welding AHW Atomic hydrogen welding NGW Narrow-gap welding CAW Constricted arc welding EGW Electro -gas welding TIG Tungsten inert-gas arc welding PMIG Plasma MIG welding MIG Metal inert-gas arc welding MAG Metal active-gas arc welding p Pulsed arc PJW Plasma jet welding sh Short arc PAW Plasma arc welding sp Spray arc PJPW Plasma jet plasma arc welding l Long arc TIG Welding Principle of TIG Welding TIG welding equipment Watercooled TIG welding torch Torch forms for TIG welding Shielding gases for welding and cutting Flow meters Flow meter for torches Effect of current and inert gas Argon consumption for TIG welding Tungsten electrodes -

Welding and Joining Guidelines
Welding and Joining Guidelines The HASTELLOY® and HAYNES® alloys are known for their good weldability, which is defined as the ability of a material to be welded and to perform satisfactorily in the imposed service environment. The service performance of the welded component should be given the utmost importance when determining a suitable weld process or procedure. If proper welding techniques and procedures are followed, high-quality welds can be produced with conventional arc welding processes. However, please be aware of the proper techniques for welding these types of alloys and the differences compared to the more common carbon and stainless steels. The following information should provide a basis for properly welding the HASTELLOY® and HAYNES® alloys. For further information, please consult the references listed throughout each section. It is also important to review any alloy- specific welding considerations prior to determining a suitable welding procedure. The most common welding processes used to weld the HASTELLOY® and HAYNES® alloys are the gas tungsten arc welding (GTAW / “TIG”), gas metal arc welding (GMAW / “MIG”), and shielded metal arc welding (SMAW / “Stick”) processes. In addition to these common arc welding processes, other welding processes such plasma arc welding (PAW), resistance spot welding (RSW), laser beam welding (LBW), and electron beam welding (EBW) are used. Submerged arc welding (SAW) is generally discouraged as this process is characterized by high heat input to the base metal, which promotes distortion, hot cracking, and precipitation of secondary phases that can be detrimental to material properties and performance. The introduction of flux elements to the weld also makes it difficult to achieve a proper chemical composition in the weld deposit. -
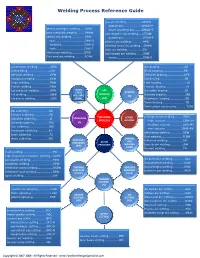
Welding Process Reference Guide
Welding Process Reference Guide gas arc welding…………………..GMAW -pulsed arc…………….……….GMAW-P atomic hydrogen welding……..AHW -short circuiting arc………..GMAW-S bare metal arc welding…………BMAW gas tungsten arc welding…….GTAW carbon arc welding……………….CAW -pulsed arc……………………….GTAW-P -gas……………………………………CAW-G plasma arc welding……………..PAW -shielded……………………………CAW-S shielded metal arc welding….SMAW -twin………………………………….CAW-T stud arc welding………………….SW electrogas welding……………….EGW submerged arc welding……….SAW Flux cord arc welding…………..FCAW -series………………………..…….SAW-S coextrusion welding……………...CEW Arc brazing……………………………..AB cold welding…………………………..CW Block brazing………………………….BB diffusion welding……………………DFW Diffusion brazing…………………….DFB explosion welding………………….EXW Dip brazing……………………………..DB forge welding…………………………FOW Flow brazing…………………………….FLB friction welding………………………FRW Furnace brazing……………………… FB hot pressure welding…………….HPW SOLID ARC Induction brazing…………………….IB STATE BRAZING WELDING Infrared brazing……………………….IRB roll welding…………………………….ROW WELDING (8) ultrasonic welding………………….USW (SSW) (AW) Resistance brazing…………………..RB Torch brazing……………………………TB Twin carbon arc brazing…………..TCAB dip soldering…………………………DS furnace soldering………………….FS WELDING OTHER electron beam welding………….EBW induction soldering……………….IS SOLDERING PROCESS WELDNG -high vacuum…………………….EBW-HV infrared soldering…………………IRS (S) -medium vacuum………………EBW-MV iron soldering……………………….INS -non-vacuum…………………….EBW-NV resistance soldering…………….RS electroslag welding……………….ESW torch soldering……………………..TS -

Tendencies in Development of Plasma-Arc Welding of Aluminium Alloys*
MAIN TENDENCIES IN DEVELOPMENT OF PLASMA-ARC WELDING OF ALUMINIUM ALLOYS* A.A. GRINYUK2, 3, V.N. KORZHIK1, 2, V.E. SHEVCHENKO1, 2, A.A. BABICH2, S.I. PELESHENKO4, V.G. CHAJKA2, A.F. TISHCHENKO2 and G.V. KOVBASENKO2 1Chinese-Ukrainian E.O. Paton Welding Institute (Guangdong General Research Institute of Industrial Technology) (Guangzhou Research Institute of Non-Ferrous Metals, PRC) 2E.O. Paton Electric Welding Institute, NASU 11 Bozhenko Str., 03680, Kiev, Ukraine. E-mail: [email protected] 3NTUU «Kiev Polytechnic Institute» 6/2 Dashavskaya Str., 03056, Kiev, Ukraine. E-mail: [email protected] 4South China University of Technology 510641, Guangzhou, PRC. E-mail: [email protected] Publications, describing the characteristic technologies of aluminium alloy welding by an arc constricted by high-velocity inert gas flow were analyzed. It is shown that plasma-arc welding (PAW) is further development of the process of nonconsumable-electrode inert-gas welding. It is established that during development of PAW of aluminium alloys, there was a transition from alternating sinusoidal current to reverse polarity direct current, and furtheron to variable polarity asymmetrical current with rectangular current waveform. A more promising direction of improvement of PAW equipment is transition from specialized power sources to modular design of PAW system, based on power sources applied for noncon- sumable electrode welding and plasma modules. Further path of improvement of the processes of aluminium alloy PAW is combined or hybrid application of several heat sources, including the constricted arc and consumable-electrode arc. In the authors’ opinion, hybrid consumable-electrode PAW with hollow anode and axial wire feed in the most promising variant.