Short Parietal Lobe Connections of the Human and Monkey Brain
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

Potential Scalp Stimulation Targets for Mental Disorders
Cao et al. J Transl Med (2021) 19:343 https://doi.org/10.1186/s12967-021-02993-1 Journal of Translational Medicine RESEARCH Open Access Potential scalp stimulation targets for mental disorders: evidence from neuroimaging studies Jin Cao, Thalia Celeste Chai‑Zhang, Yiting Huang, Maya Nicole Eshel and Jian Kong* Abstract Mental disorders widely contribute to the modern global disease burden, creating a signifcant need for improvement of treatments. Scalp stimulation methods (such as scalp acupuncture and transcranial electrical stimulation) have shown promising results in relieving psychiatric symptoms. However, neuroimaging fndings haven’t been well‑ integrated into scalp stimulation treatments. Identifying surface brain regions associated with mental disorders would expand target selection and the potential for these interventions as treatments for mental disorders. In this study, we performed large‑scale meta‑analyses separately on eight common mental disorders: attention defcit hyperactivity disorder, anxiety disorder, autism spectrum disorder, bipolar disorder, compulsive disorder, major depression, post‑ traumatic stress disorder and schizophrenia; utilizing modern neuroimaging literature to summarize disorder‑asso‑ ciated surface brain regions, and proposed neuroimaging‑based target protocols. We found that the medial frontal gyrus, the supplementary motor area, and the dorsal lateral prefrontal cortex are commonly involved in the patho‑ physiology of mental disorders. The target protocols we proposed may provide new brain targets for scalp stimulation in the treatment of mental disorders, and facilitate its clinical application. Keywords: Neuroimaging, Meta‑analysis, Scalp stimulation, Scalp acupuncture, Transcranial electrical stimulation, Mental disorder Introduction For instance, scalp acupuncture, a modern school of Mental disorders are a major component of the modern acupuncture developed on the basis of anatomical and global disease burden. -

1. Lateral View of Lobes in Left Hemisphere TOPOGRAPHY
TOPOGRAPHY T1 Division of Cerebral Cortex into Lobes 1. Lateral View of Lobes in Left Hemisphere 2. Medial View of Lobes in Right Hemisphere PARIETAL PARIETAL LIMBIC FRONTAL FRONTAL INSULAR: buried OCCIPITAL OCCIPITAL in lateral fissure TEMPORAL TEMPORAL 3. Dorsal View of Lobes 4. Ventral View of Lobes PARIETAL TEMPORAL LIMBIC FRONTAL OCCIPITAL FRONTAL OCCIPITAL Comment: The cerebral lobes are arbitrary divisions of the cerebrum, taking their names, for the most part, from overlying bones. They are not functional subdivisions of the brain, but serve as a reference for locating specific functions within them. The anterior (rostral) end of the frontal lobe is referred to as the frontal pole. Similarly, the anterior end of the temporal lobe is the temporal pole, and the posterior end of the occipital lobe the occipital pole. TOPOGRAPHY T2 central sulcus central sulcus parietal frontal occipital lateral temporal lateral sulcus sulcus SUMMARY CARTOON: LOBES SUMMARY CARTOON: GYRI Lateral View of Left Hemisphere central sulcus postcentral superior parietal superior precentral gyrus gyrus lobule frontal intraparietal sulcus gyrus inferior parietal lobule: supramarginal and angular gyri middle frontal parieto-occipital sulcus gyrus incision for close-up below OP T preoccipital O notch inferior frontal cerebellum gyrus: O-orbital lateral T-triangular sulcus superior, middle and inferior temporal gyri OP-opercular Lateral View of Insula central sulcus cut surface corresponding to incision in above figure insula superior temporal gyrus Comment: Insula (insular gyri) exposed by removal of overlying opercula (“lids” of frontal and parietal cortex). TOPOGRAPHY T3 Language sites and arcuate fasciculus. MRI reconstruction from a volunteer. central sulcus supramarginal site (posterior Wernicke’s) Language sites (squares) approximated from electrical stimulation sites in patients undergoing operations for epilepsy or tumor removal (Ojeman and Berger). -

Supplementary Tables
Supplementary Tables: ROI Atlas Significant table grey matter Test ROI # Brainetome area beta volume EG pre vs post IT 8 'superior frontal gyrus, part 4 (dorsolateral area 6), right', 0.773 17388 11 'superior frontal gyrus, part 6 (medial area 9), left', 0.793 18630 12 'superior frontal gyrus, part 6 (medial area 9), right', 0.806 24543 17 'middle frontal gyrus, part 2 (inferior frontal junction), left', 0.819 22140 35 'inferior frontal gyrus, part 4 (rostral area 45), left', 1.3 10665 67 'paracentral lobule, part 2 (area 4 lower limb), left', 0.86 13662 EG pre vs post ET 20 'middle frontal gyrus, part 3 (area 46), right', 0.934 28188 21 'middle frontal gyrus, part 4 (ventral area 9/46 ), left' 0.812 27864 31 'inferior frontal gyrus, part 2 (inferior frontal sulcus), left', 0.864 11124 35 'inferior frontal gyrus, part 4 (rostral area 45), left', 1 10665 50 'orbital gyrus, part 5 (area 13), right', -1.7 22626 67 'paracentral lobule, part 2 (area 4 lower limb), left', 1.1 13662 180 'cingulate gyrus, part 3 (pregenual area 32), right', 0.9 10665 261 'Cerebellar lobule VIIb, vermis', -1.5 729 IG pre vs post IT 16 middle frontal gyrus, part 1 (dorsal area 9/46), right', -0.8 27567 24 'middle frontal gyrus, part 5 (ventrolateral area 8), right', -0.8 22437 40 'inferior frontal gyrus, part 6 (ventral area 44), right', -0.9 8262 54 'precentral gyrus, part 1 (area 4 head and face), right', -0.9 14175 64 'precentral gyrus, part 2 (caudal dorsolateral area 6), left', -1.3 18819 81 'middle temporal gyrus, part 1 (caudal area 21), left', -1.4 14472 -

Quasi-Periodic Patterns Contribute to Functional Connectivity in the Brain
bioRxiv preprint doi: https://doi.org/10.1101/323162; this version posted May 16, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Quasi-periodic patterns contribute to functional connectivity in the brain Anzar Abbasa, Michaël Belloyb, Amrit Kashyapc, Jacob Billingsa, Maysam Nezafatic, Shella Keilholza,c a Neuroscience, Emory University, 1760 Haygood Dr NE Suite W-200, Atlanta, GA 30322, United States b Bio-Imaging Lab, University of Antwerp, Universiteitsplein 1, 2610 Wilrijk, Antwerp, Belgium c Biomedical Engineering, Emory University and Georgia Institute of Technology, 1760 Haygood Dr NE Suite W-200, Atlanta, GA 30322, United States Corresponding Author Shella Keilholz [email protected] 1760 Haygood Dr NE W-230 Atlanta, GA 30322 Highlights • Quasi-periodic patterns (QPPs) of low-frequency activity contribute to functional connectivity • The spatiotemporal pattern of QPPs differs between resting-state and task-performing individuals • QPPs account for significant functional connectivity in the DMN and TPN during rest and task performance • Changes in functional connectivity in these networks may actually reflect differences in the QPPs Declarations of Interest None !1 bioRxiv preprint doi: https://doi.org/10.1101/323162; this version posted May 16, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. -

Parietal Dysgraphia: Characterization of Abnormal Writing Stroke Sequences, Character Formation and Character Recall
Behavioural Neurology 18 (2007) 99–114 99 IOS Press Parietal dysgraphia: Characterization of abnormal writing stroke sequences, character formation and character recall Yasuhisa Sakuraia,b,∗, Yoshinobu Onumaa, Gaku Nakazawaa, Yoshikazu Ugawab, Toshimitsu Momosec, Shoji Tsujib and Toru Mannena aDepartment of Neurology, Mitsui Memorial Hospital, Tokyo, Japan bDepartment of Neurology, Graduate School of Medicine, University of Tokyo, Tokyo, Japan cDepartment of Radiology, Graduate School of Medicine, University of Tokyo, Tokyo, Japan Abstract. Objective: To characterize various dysgraphic symptoms in parietal agraphia. Method: We examined the writing impairments of four dysgraphia patients from parietal lobe lesions using a special writing test with 100 character kanji (Japanese morphograms) and their kana (Japanese phonetic writing) transcriptions, and related the test performance to a lesion site. Results: Patients 1 and 2 had postcentral gyrus lesions and showed character distortion and tactile agnosia, with patient 1 also having limb apraxia. Patients 3 and 4 had superior parietal lobule lesions and features characteristic of apraxic agraphia (grapheme deformity and a writing stroke sequence disorder) and character imagery deficits (impaired character recall). Agraphia with impaired character recall and abnormal grapheme formation were more pronounced in patient 4, in whom the lesion extended to the inferior parietal, superior occipital and precuneus gyri. Conclusion: The present findings and a review of the literature suggest that: (i) a postcentral gyrus lesion can yield graphemic distortion (somesthetic dysgraphia), (ii) abnormal grapheme formation and impaired character recall are associated with lesions surrounding the intraparietal sulcus, the symptom being more severe with the involvement of the inferior parietal, superior occipital and precuneus gyri, (iii) disordered writing stroke sequences are caused by a damaged anterior intraparietal area. -

A Practical Review of Functional MRI Anatomy of the Language and Motor Systems
REVIEW ARTICLE FUNCTIONAL A Practical Review of Functional MRI Anatomy of the Language and Motor Systems X V.B. Hill, X C.Z. Cankurtaran, X B.P. Liu, X T.A. Hijaz, X M. Naidich, X A.J. Nemeth, X J. Gastala, X C. Krumpelman, X E.N. McComb, and X A.W. Korutz ABSTRACT SUMMARY: Functional MR imaging is being performed with increasing frequency in the typical neuroradiology practice; however, many readers of these studies have only a limited knowledge of the functional anatomy of the brain. This text will delineate the locations, anatomic boundaries, and functions of the cortical regions of the brain most commonly encountered in clinical practice—specifically, the regions involved in movement and language. ABBREVIATIONS: FFA ϭ fusiform face area; IPL ϭ inferior parietal lobule; PPC ϭ posterior parietal cortex; SMA ϭ supplementary motor area; VOTC ϭ ventral occipitotemporal cortex his article serves as a review of the functional areas of the brain serving to analyze spatial position and the ventral stream working Tmost commonly mapped during presurgical fMRI studies, to identify what an object is. Influenced by the dorsal and ventral specifically targeting movement and language. We have compiled stream model of vision, Hickok and Poeppel2 hypothesized a sim- what we hope is a useful, easily portable, and concise resource that ilar framework for language. In this model, the ventral stream, or can be accessible to radiologists everywhere. We begin with a re- lexical-semantic system, is involved in sound-to-meaning map- view of the language-processing system. Then we describe the pings associated with language comprehension and semantic ac- gross anatomic boundaries, organization, and function of each cess. -

1 a Taxonomy of the Brain's White Matter
A taxonomy of the brain’s white matter: Twenty-one major tracts for the twenty-first century Daniel N. Bullock1, Elena A. Hayday2, Mark D. Grier2, *Wei Tang1,3, *Franco Pestilli4, *Sarah R. Heilbronner2 *These authors share senior author contribution 1. Department of Psychological and Brain Sciences, Program in Neuroscience, Indiana University Bloomington, Bloomington, IN USA 47405 2. Department of Neuroscience, University of Minnesota, Minneapolis, MN USA 55455 3. Department of Computer Science, Indiana University Bloomington, Bloomington, IN USA 47408 4. Department of Psychology, The University of Texas at Austin, Austin, TX USA 78712 Correspondence: Sarah R. Heilbronner, PhD 2-164 Jackson Hall 321 Church St SE Minneapolis, MN 55455 772-285-7021 [email protected] Acknowledgements: SRH was supported by NIH R01MH118257 (SRH); MDG was supported by NIDA T32DA007234 to P. Mermelstein (UMN); DNB was supported by NIMH 5T32MH103213-05 to W. Hetrick (IU); FP was supported by NSF IIS-1636893, NSF BCS-1734853, NSF IIS-1912270, NIH NIBIB 1-R01-EB029272- 01, and a Microsoft Investigator Fellowship. Conflicts: SRH has received teaching fees from Medtronic, Inc. Remaining authors have no conflicts to disclose. 1 Abstract The functional and computational properties of brain areas are determined, in large part, by their connectivity profiles. Advances in neuroimaging and network neuroscience allow us to characterize the human brain noninvasively and in vivo, but a comprehensive understanding of the human brain demands an account of the anatomy of brain connections. Long-range anatomical connections are instantiated by white matter and organized into tracts. Here, we aim to characterize the connections, morphology, traversal, and functions of the major white matter tracts in the brain. -

The Effects of Pharmacological Opioid Blockade on Neural Measures of Drug Cue-Reactivity in Humans
Neuropsychopharmacology (2016) 41, 2872–2881 © 2016 American College of Neuropsychopharmacology. All rights reserved 0893-133X/16 www.neuropsychopharmacology.org The Effects of Pharmacological Opioid Blockade on Neural Measures of Drug Cue-Reactivity in Humans 1 2 ,1,2 Kelly E Courtney , Dara G Ghahremani and Lara A Ray* 1 2 Department of Psychology, University of California, Los Angeles, CA, USA; Department of Psychiatry and Biobehavioral Sciences, University of California, Los Angeles, CA, USA Interactions between dopaminergic and opioidergic systems have been implicated in the reinforcing properties of drugs of abuse. The present study investigated the effects of opioid blockade, via naltrexone, on functional magnetic resonance imaging (fMRI) measures during methamphetamine cue-reactivity to elucidate the role of endogenous opioids in the neural systems underlying drug craving. To investigate = = = this question, non-treatment seeking individuals with methamphetamine use disorder (N 23; 74% male, mean age 34.70 (SD 8.95)) were recruited for a randomized, placebo controlled, within-subject design and underwent a visual methamphetamine cue-reactivity task during two blood-oxygen-level dependent (BOLD) fMRI sessions following 3 days of naltrexone (50 mg) and matched time for placebo. fMRI analyses tested naltrexone-induced differences in BOLD activation and functional connectivity during cue processing. The results showed that naltrexone administration reduced cue-reactivity in sensorimotor regions and related to altered functional connectivity of dorsal striatum, ventral tegmental area, and precuneus with frontal, visual, sensory, and motor-related regions. Naltrexone also weakened the associations between subjective craving and precuneus functional connectivity with sensorimotor regions and strengthened the associations between subjective craving and dorsal striatum and precuneus connectivity with frontal regions. -
Core-Example1.Pdf
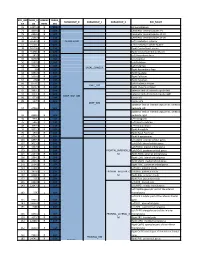
ROI_IND NUM_V HEMISP TISSUE_ SUBGROUP_0 SUBGROUP_1 SUBGROUP_2 ROI_NAME EX OX HERE SEG 95 12871.8 B WM corpus callosum 71 4899.8 B GM Cerebellar Vermal Lobules I-V 73 2858.8 B GM Cerebellar Vermal Lobules VIII-X 72 2266.9 B GM Cerebellar Vermal Lobules VI-VII 39 54582.6 L GM CEREBELLUM Left Cerebellum Exterior 41 15500.7 L WM Left Cerebellum White Matter 38 54379.4 R GM Right Cerebellum Exterior 40 15458.7 R WM Right Cerebellum White Matter 30 585.9 L GM Left Accumbens Area 37 3578.9 L GM Left Caudate 56 1597.6 L GM Left Pallidum 58 4942.3 L GM Left Putamen BASAL_GANGLIA 23 526 R GM Right Accumbens Area 36 3651.5 R GM Right Caudate 55 1638.8 R GM Right Pallidum 57 4726 R GM Right Putamen 60 8574.1 L GM Left Thalamus Proper DEEP_GM 59 8256.3 R GM Right Thalamus Proper 92 2887.7 L WM anterior limb of internal capsule left 91 3393.3 R WM anterior limb of internal capsule right DEEP_WM_GM 90 673.6 L WM fornix left 89 517.5 R WM fornix right DEEP_WM posterior limb of internal capsule inc. cerebral 94 2416.3 L WM peduncle left posterior limb of internal capsule inc. cerebral 93 2480.5 R WM peduncle right 32 993.7 L GM Left Amygdala 75 586.5 L GM Left Basal Forebrain 48 3597.7 L GM Left Hippocampus 31 1021.3 R GM Right Amygdala 76 593.1 R GM Right Basal Forebrain 47 3704.7 R GM Right Hippocampus 105 1897.7 L GM Left AOrG anterior orbital gyrus 137 3015.9 L GM Left LOrG lateral orbital gyrus 147 4637.3 L GM Left MOrG medial orbital gyrus 179 2915.7 L GM FRONTAL_INFERIOR_G Left POrG posterior orbital gyrus 104 2244.9 R GM M Right AOrG anterior orbital -

Task Positive and Default Mode Networks During a Working Memory in Children with Primary Monosymptomatic Nocturnal Enuresis and Healthy Controls
nature publishing group Articles Clinical Investigation Task positive and default mode networks during a working memory in children with primary monosymptomatic nocturnal enuresis and healthy controls Kaihua Zhang1, Jun Ma2, Du Lei1,3, Mengxing Wang1, Jilei Zhang1 and Xiaoxia Du1 BACKGROUND: Nocturnal enuresis is a common develop- event-related brain potentials indicated that maturational mental disorder in children, and primary monosymptomatic delays in central nervous system development are indicators nocturnal enuresis (PMNE) is the dominant subtype. of PMNE pathogenesis (5–8). Primary nocturnal enuresis chil- METHODS: This study investigated brain functional abnor- dren show a higher prevalence of all sleep disturbances (9). malities that are specifically related to working memory in Furthermore, the prevalence of fine motor coordination and children with PMNE using function magnetic resonance imag- visuomotor integration were abnormal in prepubertal children ing (fMRI) in combination with an n-back task. Twenty children with PMNE. These studies suggested that PMNE should not be with PMNE and 20 healthy children, group-matched for age considered as a voiding disorder alone (10). and sex, participated in this experiment. Magnetic resonance imaging (MRI) techniques, such as RESULTS: Several brain regions exhibited reduced activation structural MRI, functional MRI (fMRI), and diffusion MRI, during the n-back task in children with PMNE, including the provide efficient, feasible and noninvasive methods to inves- right precentral gyrus and the right inferior parietal lobule tigate the biological mechanisms of incontinence. Several extending to the postcentral gyrus. Children with PMNE exhib- studies reported alterations in brain functions in patients with ited decreased cerebral activation in the task-positive network, urgency and urge incontinence using fMRI (11,12). -

Human Brain – 4 Part 1313104
V1/17-2016© Human Brain – 4 Part 1313104 Left Hemisphere of Cerebrum, Lateral View 1. 37. Cingulate Gyrus a. Motor Area (Somatomotor Cortex) 38. Cingulate Sulcus b. Sensory Area (Somatosensory Cortex) 39. Subfrontal Part of Cingulate Sulcus 2. Frontal Lobe 40. Sulcus of Corpus Callosum 3. Temporal Lobe 41. Body of Corpus Callosum 4. Parietal Lobe 42. Genu of Corpus Callosum 5. Occipital Lobe 43. Splenium of Corpus Callosum 6. Left Hemisphere of Cerebellum 44. Septum Pellucidum 7. Superior Frontal Gyrus 45. Body of Fornix 8. Middle Frontal Gyrus 46. Anterior Commissure 9. Inferior Frontal Gyrus 47. Intermediate Mass 10. Precentral Gyrus (Somatomotor Cortex) 48. Posterior Commissure 11. Postcentral Gyrus (Somatosensory Cortex) 49. Thalamus 12. Superior Parietal Lobule 50. Foramen of Monro 13. Inferior Parietal Lobule 51. Hypothalamic Sulcus 14. Lateral Occipital Gyri 52. Lamina Terminalis 15. Superior Temporal Gyrus 53. Optic Chiasm 16. Middle Temporal Gyrus 54. Pineal Gland 17. Inferior Temporal Gyrus 55. Pineal Recess 18. Superior Frontal Sulcus 56. Corpora Quadrigemina 19. Pre-central Sulcus 57. Sylvian Aqueduct 20. Central Sulcus 58. Superior Medullary Vellum 21. Post-central Sulcus 59. Fourth Ventricle 22. Intraparietal Sulcus 60. Central Canal of Medulla Oblongata 23. Transverse Occipital Sulcus 61. Arbor Vitae 24. Lateral Occipital Sulcus 62. Vermis of Cerebellum 25. Parieto-occipital Sulcus 63. Medulla Oblongata 26. Superior Temporal Sulcus 64. Medial Longitudinal Fasciculus 27. Middle Temporal Sulcus 65. Pons 28. Lateral Cerebral Sulcus (Sylvian Fissure) 66. Longitudinal Fibers of Pons 29. Anterior Horizontal Ramus of Sylvian Fissure 67. Cerebral Peduncle 30. Anterior Ascending Ramus of Sylvian Fissure 68. Tuber Cinereum 31.