Development of the Reproductive Organs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

The Reproductive System
27 The Reproductive System PowerPoint® Lecture Presentations prepared by Steven Bassett Southeast Community College Lincoln, Nebraska © 2012 Pearson Education, Inc. Introduction • The reproductive system is designed to perpetuate the species • The male produces gametes called sperm cells • The female produces gametes called ova • The joining of a sperm cell and an ovum is fertilization • Fertilization results in the formation of a zygote © 2012 Pearson Education, Inc. Anatomy of the Male Reproductive System • Overview of the Male Reproductive System • Testis • Epididymis • Ductus deferens • Ejaculatory duct • Spongy urethra (penile urethra) • Seminal gland • Prostate gland • Bulbo-urethral gland © 2012 Pearson Education, Inc. Figure 27.1 The Male Reproductive System, Part I Pubic symphysis Ureter Urinary bladder Prostatic urethra Seminal gland Membranous urethra Rectum Corpus cavernosum Prostate gland Corpus spongiosum Spongy urethra Ejaculatory duct Ductus deferens Penis Bulbo-urethral gland Epididymis Anus Testis External urethral orifice Scrotum Sigmoid colon (cut) Rectum Internal urethral orifice Rectus abdominis Prostatic urethra Urinary bladder Prostate gland Pubic symphysis Bristle within ejaculatory duct Membranous urethra Penis Spongy urethra Spongy urethra within corpus spongiosum Bulbospongiosus muscle Corpus cavernosum Ductus deferens Epididymis Scrotum Testis © 2012 Pearson Education, Inc. Anatomy of the Male Reproductive System • The Testes • Testes hang inside a pouch called the scrotum, which is on the outside of the body -

Cranial Cavitry
Embryology Endo, Energy, and Repro 2017-2018 EMBRYOLOGY OF THE REPRODUCTIVE SYSTEM Janine Prange-Kiel, Ph.D. Office: L1.106, Phone: 83117 Email: [email protected] LEARNING OBJECTIVES: • Name the structures in kidney development that contribute to the development of the reproductive organs. • Predict how the presence or absence of the Y chromosome and the expression of the SRY gene would influence the development of the gonads. • Predict how the presence or absence of testosterone, dihydrotestosterone, and anit- Mullerian hormone would influence the development of the genital ducts and indifferent primordia of the external genitalia. I. Introduction In general, the function of the genital (reproductive) system in males and females is the formation, nurture, and transport of germ cells. In females, an additional function is to provide the proper milieu for the fetal development after conception. Like the urinary system, the genital system derives from intermediate mesoderm. The development of these two systems is tightly interwoven as structures that develop as parts of the urinary system gain function in the genital system. In the adult, the sexual organs differ between males and females. The early genital system, however, is similar in both sexes, and the sexual differentiation of this initially indifferent, bipotential system starts only in the seventh week of embryonic development. The details on how sexual differentiation is determined will be discussed below, but it is worth mentioning here that irregularities in this process result in disorders of sexual differentiation (DSDs). DSDs occur in approximately 1 in 4,500 live births and will be discussed in a separate lecture. -

On Cysts of the Prepuce and Raphé, with an Illustrative Case
ON CYSTS OF THE PREPUCE AND RAPHE, WITH AN ILLUSTRATIVE CASE* By GEO. HENRY EDINGTON, M.D., M.R.C.S. Eng., Surgeon to the Central Dispensary, and Extra Surgeon to the Dispensary of the Western Infirmary, Glasgow. Cysts of the prepuce receive but scant notice in the general text-books of surgery, and it is partly on this account that I now record the following case. I have been led, however, to do so also from the fact that recently cysts in this region have been engaging the minds of some writers in connection with their probable origin in a congenital abnormality. I shall first notice a case which has come under my own observation, and then refer to the literature of the subject. Willie D., aged 1 year, was brought to the Central Dispensary on 27th October, 1897, on account of his being the subject of " phimosis, accompanied by a small lump" at the distal extremity of the prepuce. This lump was first observed when he was 3 months old, and it increased in size till he reached the age of 6 months, since when no alteration in dimension had been noticed. It was stated, however, that, after ceasing to enlarge, the swelling had become harder than when first noticed. The prepuce (Fig. 1, p. 423), which was long, presented on inspection a spherical swelling on the under aspect of the free margin, with the antero-posterior vertical meridian corres- * Paper read and specimen shown at the meeting of the Glasgow Pathological and Clinical Society, 11th April, 1898. -

Short Communication Clinical and Pathological
Reprod Dom Anim 45, 368–372 (2010); doi: 10.1111/j.1439-0531.2009.01495.x ISSN 0936-6768 Short Communication Clinical and Pathological Findings of a Sac-like Formation in the Tunica Vaginalis of a Nelore (Bos indicus) Bull J Chaco´n1 and A Berrocal2,* 1Section of Andrology, Research Program on Applied Animal Andrology; 2Department of Pathology, School of Veterinary Medicine, Universidad, Nacional (UNA), Heredia, Costa Rica Contents and abnormalities in the ejaculate compared with A seven-month-old purebred Nelore calf was diagnosed with a normal bulls (Chaco´n et al. 1999; Chaco´n 2001). bilateral finger-shaped swelling although more prominent at Regarding the tunica vaginalis in the bull, abnormal- the left side of the scrotum, located longitudinal and parallel to ities reported in this serosa are mainly limited to epididymis corpus. The finding was present from 7 months of conditions such as hydrocele and adherences within age up to castration (performed at 25 months of age). Scrotal tunica layers secondary to orchitis. Blom and Christen- circumference, testicular and epididymis consistency and sen (1958) described also the presence of cyst like symmetry as well as seminal quality were normal during the formations (paradidymis), in the funiculus spermaticus follow-up period. The ultrasonographic appearance of the scrotal wall, pampiniform plexus, gonad and epididymis was (mesorchium), presumable derived from remnants of the normal. However, an anechoic region surrounded by a wall mesonephric duct. It is unknown that sac-like forma- forming a sac-like structure with a blind end at its dorsal pole tions in the tunica vaginalis of the bull or any other was seen on the swelling area. -
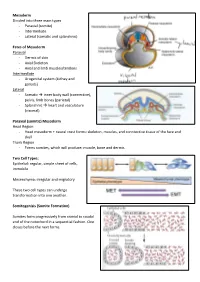
Mesoderm Divided Into Three Main Types - Paraxial (Somite) - Intermediate - Lateral (Somatic and Splanchnic)
Mesoderm Divided into three main types - Paraxial (somite) - Intermediate - Lateral (somatic and splanchnic) Fates of Mesoderm Paraxial - Dermis of skin - Axial Skeleton - Axial and limb muscles/tendons Intermediate - Urogenital system (kidney and gonads) Lateral - Somatic inner body wall (connective), pelvis, limb bones (parietal) - Splanchnic heart and vasculature (visceral) Paraxial (somitic) Mesoderm Head Region - Head mesoderm + neural crest forms: skeleton, muscles, and conntective tissue of the face and skull Trunk Region - Forms somites, which will produce: muscle, bone and dermis Two Cell Types: Epithelial: regular, simple sheet of cells, immobile Mesenchyma: irregular and migratory These two cell types can undergo transformation into one another. Somitogenisis (Somite Formation) Somites form progressively from cranial to caudal end of the notochord in a sequential fashion. One closes before the next forms. Somite Differentiation The somite splits into the epithelial dermamyotome (dermis/muscle) and the messenchymal sclerotome (skeletal). The somite is all paraxial mesoderm. Somite location determines the fate of its associates derma/myo/sclerotomes. Intermediate Mesoderm Urogenital system: - Kidneys - Gonads - Reproductive Duct Systems Runs alongside the paraxial mesoderm. Urogenital System Along mesonephric duct: - Pronephros, mesonephros, and metanephros - Pronephros fall away as gonad develops on ventral-medial side of mesonephros. - Metanephrogenic mesenchyme gives rise to kidney. The mesonephric duct will become the Wolffian duct forming at the nephric bud. The Mullerian duct forms via an invagination on the dorsal side of the nephric duct. The gonad will degenerate one of the two ducts depending on the hormones it produces. XX degenerates Wolffian duct – no testosterone, anti-Mullerian hormone (AMH) not produced, and Mullerian duct can develop in addition to female reproductive organs (ovaries, vagina) XY degenerates Mullerian duct – testosterone, AMH produced, Wolffian duct continues as male reproductive organs (testes, penis) develop. -

Renal Development
RENAL DEVELOPMENT Jon Barasch M.D., Ph.D. Telephone: 305-1890 e-mail: [email protected] SUGGESTED READING: Larsen, 3rd edition, pp 265 (first three paragraphs) - 266, 268-276 and figure 10-10 LEARNING OBJECTIVES: You should be able to: 1. Describe the three kidneys that are produced during development and know what happens to each one. 2. Explain what is meant by ‘reciprocal induction’ and why it poses problems in interpreting experiments in developing kidney. 3. Describe the stages of nephron formation from the renal vesicle. 4. Discuss the regulators of mesenchymal to epithelial transition in the intermediate mesoderm and metanephric mesenchyme and name three molecules mediating conversion. 5. Describe branching morphogenesis and name the three patterns in the developing metanephros. 6. Discuss three key important ligands and their receptors. 7. Discuss the classification of congenital renal abnormalities that are associated with urological abnormalities and the possible underlying mechanisms for their association. SUMMARY: The urogenital system derives from mesenchymal cells by a process of conversion to epithelia. The development of the kidney relies on three mechanisms of epithelial morphogenesis. 1. Some newborn epithelia migrate extensively (Wolffian duct), 2. some undergo branching morphogenesis (ureteric bud) and 3. some produce highly segmented tubules (nephrons). GLOSSARY: Angiotensin II: your favorite vasoconstrictor and regulator of proximal tubule reclamation of NaCl and water by receptor type 1. Receptor type-2 modulates cell growth and seems to play a role in congenital abnormalities. Arcade: a tubule of ureteric bud that induces a few nephrons simultaneously. The nephrons join to a common drainage called a connecting tubule that feeds into the ureteric’s collecting duct. -

1- Development of Female Genital System
Development of female genital systems Reproductive block …………………………………………………………………. Objectives : ✓ Describe the development of gonads (indifferent& different stages) ✓ Describe the development of the female gonad (ovary). ✓ Describe the development of the internal genital organs (uterine tubes, uterus & vagina). ✓ Describe the development of the external genitalia. ✓ List the main congenital anomalies. Resources : ✓ 435 embryology (males & females) lectures. ✓ BRS embryology Book. ✓ The Developing Human Clinically Oriented Embryology book. Color Index: ✓ EXTRA ✓ Important ✓ Day, Week, Month Team leaders : Afnan AlMalki & Helmi M AlSwerki. Helpful video Focus on female genital system INTRODUCTION Sex Determination - Chromosomal and genetic sex is established at fertilization and depends upon the presence of Y or X chromosome of the sperm. - Development of female phenotype requires two X chromosomes. - The type of sex chromosomes complex established at fertilization determine the type of gonad differentiated from the indifferent gonad - The Y chromosome has testis determining factor (TDF) testis determining factor. One of the important result of fertilization is sex determination. - The primary female sexual differentiation is determined by the presence of the X chromosome , and the absence of Y chromosome and does not depend on hormonal effect. - The type of gonad determines the type of sexual differentiation in the Sexual Ducts and External Genitalia. - The Female reproductive system development comprises of : Gonad (Ovary) , Genital Ducts ( Both male and female embryo have two pair of genital ducts , They do not depend on ovaries or hormones ) and External genitalia. DEVELOPMENT OF THE GONADS (ovaries) - Is Derived From Three Sources (Male Slides) 1. Mesothelium 2. Mesenchyme 3. Primordial Germ cells (mesodermal epithelium ) lining underlying embryonic appear among the Endodermal the posterior abdominal wall connective tissue cell s in the wall of the yolk sac). -

Chronic Hazard Advisory Panel on Phthalates and Phthalate Alternatives
Report to the U.S. Consumer Product Safety Commission by the CHRONIC HAZARD ADVISORY PANEL ON PHTHALATES AND PHTHALATE ALTERNATIVES July 2014 U.S. Consumer Product Safety Commission Directorate for Health Sciences Bethesda, MD 20814 Chronic Hazard Advisory Panel on Phthalates and Phthalate Alternatives Chris Gennings, Ph.D. Virginia Commonwealth University Richmond, VA Russ Hauser, M.D., Sc.D., M.P.H. Harvard School of Public Health Boston, MA Holger M. Koch, Ph.D. Ruhr University Bochum, Germany Andreas Kortenkamp, Ph.D. Brunel University London, United Kingdom Paul J. Lioy, Ph.D. Robert Wood Johnson Medical School Piscataway, NJ Philip E. Mirkes, Ph.D. University of Washington (retired) Seattle, WA Bernard A. Schwetz, D.V.M., Ph.D. Department of Health and Human Services (retired) Washington, DC TABLE OF CONTENTS LIST OF TABLES ....................................................................................................................... iv LIST OF FIGURES ..................................................................................................................... vi ABBREVIATIONS ..................................................................................................................... vii 1 Executive Summary .............................................................................................................. 1 2 Background and Strategy................................................................................................... 11 2.1 Introduction and Strategy Definition ........................................................................... -

The Importance of the Gubernaculum in Testicular Migration During the Human Fetal Period ______
Vol. 40 (6): 722-729, November - December, 2014 REVIEW ARTICLE doi: 10.1590/S1677-5538.IBJU.2014.06.02 The importance of the gubernaculum in testicular migration during the human fetal period _______________________________________________ Luciano A. Favorito1, Suelen F. Costa1, Helce R. Julio-Junior1, Francisco J. B. Sampaio1 1Urogenital Research Unit, State University of Rio de Janeiro, Brazil ABSTRACT ARTICLE INFO ______________________________________________________________ ______________________ Objectives: The objective of this review is to study the role of the gubernaculum in the Key words: testicular migration process during the human fetal period. Testicular Diseases; Materials and Methods: We performed a descriptive review of the literature about the Cryptorchidism; Embryology; role of the gubernaculum in testicular migration during the human fetal period. Fetus; Testis Results: In the first phase of testicular migration, the gubernaculum enlarges to hold the testis near the groin and in the second phase the gubernaculum migrates across Int Braz J Urol. 2014; 40: 722-9 the pubic region to reach the scrotum. The proximal portion of the gubernaculum is attached to the testis and epididymis and the presence of multiple insertions in the dis- _____________________ tal gubernaculum is extremely rare. The presence of muscle and nerves in the human gubernaculum is very poor. The gubernaculum of patients with cryptorchidism has Submitted for publication: more fibrous tissue and less collagen and when the patients are submitted to hormonal June 20, 2014 _____________________ treatment, the gubernaculum components alter significantly. Conclusions: The gubernaculum presents significant structural modifications during Accepted after revision: testicular migration in human fetuses. October 31, 2014 INTRODUCTION gubernaculum’s development (1, 11). -

Unit 3 Embryo Questions
Unit 3 Embryology Clinically Oriented Anatomy (COA) Texas Tech University Health Sciences Center Created by Parker McCabe, Fall 2019 parker.mccabe@@uhsc.edu Solu%ons 1. B 11. A 21. D 2. C 12. B 22. D 3. C 13. E 23. D 4. B 14. D 24. A 5. E 15. C 25. D 6. C 16. B 26. B 7. D 17. E 27. C 8. B 18. A 9. C 19. C 10. D 20. B Digestive System 1. Which of the following structures develops as an outgrowth of the endodermal epithelium of the upper part of the duodenum? A. Stomach B. Pancreas C. Lung buds D. Trachea E. Esophagus Ques%on 1 A. Stomach- Foregut endoderm B. Pancreas- The pancreas, liver, and biliary apparatus all develop from outgrowths of the endodermal epithelium of the upper part of the duodenum. C. Lung buds- Foregut endoderm D. Trachea- Foregut endoderm E. Esophagus- Foregut endoderm 2. Where does the spleen originate and then end up after the rotation of abdominal organs during fetal development? A. Ventral mesentery à left side B. Ventral mesentery à right side C. Dorsal mesentery à left side D. Dorsal mesentery à right side E. It does not relocate Question 2 A. Ventral mesentery à left side B. Ventral mesentery à right side C. Dorsal mesentery à left side- The spleen and dorsal pancreas are embedded within the dorsal mesentery (greater omentum). After rotation, dorsal will go to the left side of the body and ventral will go to the right side of the body (except for the ventral pancreas). -

The Incidental Demonst Ation of Mesonephric Duct Remnants Du Ing Hysterosalpingogram
ACTA MËDICA PORTUGUESA 1991; 4:35-36 CASO CLÍNICO THE INCIDENTAL DEMONST ATION OF MESONEPHRIC DUCT REMNANTS DU ING HYSTEROSALPINGOGRAM A. MIGUEL DE FRANÇA MARTINS, Z. GRAUBARD, F.V.D. MERWE, S. CARTOON Department of Obstetrics and Gynaecology and Radiology. J.C. Strijdom Hospital and the University of the Witwatersrand. SUMMARY Unilateral opacification of the Ieft mesonephric duct (Gartner’s duct) was observed during Hystero salpingography. Embryological and anatomical explanation for this phenomenon and a review of the literature are presented. RESUMO Demonstração acidental do canal de Gartner durante bisterosalpingografia Observou-se durante uma histerossalpingografia a opacificação do canal mesonefrico à esquerda, (Canal de Gartner). Fazem-se algumas considerações anatómicas e embriológicas acerca de tal defeito embriológico bem como uma revisão da literatura. EMBRYOLOGY AND ANATOMY portion of the right failopian tube ended abruptly and there was non-fiuing of the left faliopian tube. The female genital organs arise from the paramesonephric Venous intravasation occurred with delineation of the ute (Müllerian) ducts. The cranial part of each duct becomes the rine venous network and i!iac vem on the left side. In addi faliopian tube, the intermediate parts fuse to a varying tion, a long beaded structure passing inferiorly, lateral to the degree to form the uterus and the caudal portions unite and vagina was filled via a narrow neck at the leve! of the inter contribute to the development of part of the vagina 1,2• na! os of the cervix. The mesonephric (Wolffian) duct disappears except for a The following view (Fig. 1) shows this structure passing few remnants to be found in the broad ligament, uterine antero, inferiorly towards the externa! genita!ia.