Cranial Cavitry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

Comparative Gene Expression Analysis of Genital Tubercle Development Reveals a Putative Appendicular Wnt7 Network for the Epidermal Differentiation
Developmental Biology 344 (2010) 1071–1087 Contents lists available at ScienceDirect Developmental Biology journal homepage: www.elsevier.com/developmentalbiology Genomes & Developmental Control Comparative gene expression analysis of genital tubercle development reveals a putative appendicular Wnt7 network for the epidermal differentiation Han Sheng Chiu a, John C. Szucsik b, Kylie M. Georgas a, Julia L. Jones c, Bree A. Rumballe a, Dave Tang a, Sean M. Grimmond a, Alfor G. Lewis b, Bruce J. Aronow b,c, James L. Lessard b, Melissa H. Little a,⁎ a Institute for Molecular Bioscience, The University of Queensland, St. Lucia 4072, Australia b Division of Developmental Biology, Cincinnati Children's Hospital Research Foundation, Cincinnati, OH 45229, USA c Division of Biomedical Informatics, Cincinnati Children's Hospital Research Foundation, Cincinnati, OH 45229, USA article info abstract Article history: Here we describe the first detailed catalog of gene expression in the developing lower urinary tract (LUT), Received for publication 30 November 2009 including epithelial and mesenchymal portions of the developing bladder, urogenital sinus, urethra, and Revised 23 April 2010 genital tubercle (GT) at E13 and E14. Top compartment-specific genes implicated by the microarray data Accepted 15 May 2010 were validated using whole-mount in situ hybridization (ISH) over the entire LUT. To demonstrate the Available online 24 May 2010 potential of this resource to implicate developmentally critical features, we focused on gene expression Keywords: patterns and pathways in the sexually indeterminate, androgen-independent GT. GT expression patterns Genital tubercle development reinforced the proposed similarities between development of GT, limb, and craniofacial prominences. Lower urinary tract development Comparison of spatial expression patterns predicted a network of Wnt7a-associated GT-enriched epithelial Gene expression genes, including Gjb2, Dsc3, Krt5, and Sostdc1. -
The Reproductive System
27 The Reproductive System PowerPoint® Lecture Presentations prepared by Steven Bassett Southeast Community College Lincoln, Nebraska © 2012 Pearson Education, Inc. Introduction • The reproductive system is designed to perpetuate the species • The male produces gametes called sperm cells • The female produces gametes called ova • The joining of a sperm cell and an ovum is fertilization • Fertilization results in the formation of a zygote © 2012 Pearson Education, Inc. Anatomy of the Male Reproductive System • Overview of the Male Reproductive System • Testis • Epididymis • Ductus deferens • Ejaculatory duct • Spongy urethra (penile urethra) • Seminal gland • Prostate gland • Bulbo-urethral gland © 2012 Pearson Education, Inc. Figure 27.1 The Male Reproductive System, Part I Pubic symphysis Ureter Urinary bladder Prostatic urethra Seminal gland Membranous urethra Rectum Corpus cavernosum Prostate gland Corpus spongiosum Spongy urethra Ejaculatory duct Ductus deferens Penis Bulbo-urethral gland Epididymis Anus Testis External urethral orifice Scrotum Sigmoid colon (cut) Rectum Internal urethral orifice Rectus abdominis Prostatic urethra Urinary bladder Prostate gland Pubic symphysis Bristle within ejaculatory duct Membranous urethra Penis Spongy urethra Spongy urethra within corpus spongiosum Bulbospongiosus muscle Corpus cavernosum Ductus deferens Epididymis Scrotum Testis © 2012 Pearson Education, Inc. Anatomy of the Male Reproductive System • The Testes • Testes hang inside a pouch called the scrotum, which is on the outside of the body -

MR Imaging of Vaginal Morphology, Paravaginal Attachments and Ligaments
MR imaging of vaginal morph:ingynious 05/06/15 10:09 Pagina 53 Original article MR imaging of vaginal morphology, paravaginal attachments and ligaments. Normal features VITTORIO PILONI Iniziativa Medica, Diagnostic Imaging Centre, Monselice (Padova), Italy Abstract: Aim: To define the MR appearance of the intact vaginal and paravaginal anatomy. Method: the pelvic MR examinations achieved with external coil of 25 nulliparous women (group A), mean age 31.3 range 28-35 years without pelvic floor dysfunctions, were compared with those of 8 women who had cesarean delivery (group B), mean age 34.1 range 31-40 years, for evidence of (a) vaginal morphology, length and axis inclination; (b) perineal body’s position with respect to the hymen plane; and (c) visibility of paravaginal attachments and lig- aments. Results: in both groups, axial MR images showed that the upper vagina had an horizontal, linear shape in over 91%; the middle vagi- na an H-shape or W-shape in 74% and 26%, respectively; and the lower vagina a U-shape in 82% of cases. Vaginal length, axis inclination and distance of perineal body to the hymen were not significantly different between the two groups (mean ± SD 77.3 ± 3.2 mm vs 74.3 ± 5.2 mm; 70.1 ± 4.8 degrees vs 74.04 ± 1.6 degrees; and +3.2 ± 2.4 mm vs + 2.4 ± 1.8 mm, in group A and B, respectively, P > 0.05). Overall, the lower third vaginal morphology was the less easily identifiable structure (visibility score, 2); the uterosacral ligaments and the parau- rethral ligaments were the most frequently depicted attachments (visibility score, 3 and 4, respectively); the distance of the perineal body to the hymen was the most consistent reference landmark (mean +3 mm, range -2 to + 5 mm, visibility score 4). -

Short Communication Clinical and Pathological
Reprod Dom Anim 45, 368–372 (2010); doi: 10.1111/j.1439-0531.2009.01495.x ISSN 0936-6768 Short Communication Clinical and Pathological Findings of a Sac-like Formation in the Tunica Vaginalis of a Nelore (Bos indicus) Bull J Chaco´n1 and A Berrocal2,* 1Section of Andrology, Research Program on Applied Animal Andrology; 2Department of Pathology, School of Veterinary Medicine, Universidad, Nacional (UNA), Heredia, Costa Rica Contents and abnormalities in the ejaculate compared with A seven-month-old purebred Nelore calf was diagnosed with a normal bulls (Chaco´n et al. 1999; Chaco´n 2001). bilateral finger-shaped swelling although more prominent at Regarding the tunica vaginalis in the bull, abnormal- the left side of the scrotum, located longitudinal and parallel to ities reported in this serosa are mainly limited to epididymis corpus. The finding was present from 7 months of conditions such as hydrocele and adherences within age up to castration (performed at 25 months of age). Scrotal tunica layers secondary to orchitis. Blom and Christen- circumference, testicular and epididymis consistency and sen (1958) described also the presence of cyst like symmetry as well as seminal quality were normal during the formations (paradidymis), in the funiculus spermaticus follow-up period. The ultrasonographic appearance of the scrotal wall, pampiniform plexus, gonad and epididymis was (mesorchium), presumable derived from remnants of the normal. However, an anechoic region surrounded by a wall mesonephric duct. It is unknown that sac-like forma- forming a sac-like structure with a blind end at its dorsal pole tions in the tunica vaginalis of the bull or any other was seen on the swelling area. -

ANA214: Systemic Embryology
ANA214: Systemic Embryology ISHOLA, Azeez Olakune [email protected] Anatomy Department, College of Medicine and Health Sciences Outline • Organogenesis foundation • Urogenital system • Respiratory System • Kidney • Larynx • Ureter • Trachea & Bronchi • Urinary bladder • Lungs • Male urethra • Female urethra • Cardiovascular System • Prostate • Heart • Uterus and uterine tubes • Blood vessels • Vagina • Fetal Circulation • External genitalia • Changes in Circulation at Birth • Testes • Gastrointestinal System • Ovary • Mouth • Nervous System • Pharynx • Neurulation • GI Tract • Neural crests • Liver, Spleen, Pancreas Segmentation of Mesoderm • Start by 17th day • Under the influence of notochord • Cells close to midline proliferate – PARAXIAL MESODERM • Lateral cells remain thin – LATERAL PLATE MESODERM • Somatic/Parietal mesoderm – close to amnion • Visceral/Splanchnic mesoderm – close to yolk sac • Intermediate mesoderm connects paraxial and lateral mesoderm Paraxial Mesoderm • Paraxial mesoderm organized into segments – SOMITOMERES • Occurs in craniocaudal sequence and start from occipital region • 1st developed by day 20 (3 pairs per day) – 5th week • Gives axial skeleton Intermediate Mesoderm • Differentiate into Urogenital structures • Pronephros, mesonephros Lateral Plate Mesoderm • Parietal mesoderm + ectoderm = lateral body wall folds • Dermis of skin • Bones + CT of limb + sternum • Visceral Mesoderm + endoderm = wall of gut tube • Parietal mesoderm surrounding cavity = pleura, peritoneal and pericardial cavity • Blood & -

Development of the Human Penis and Clitoris
Differentiation xxx (xxxx) xxx–xxx Contents lists available at ScienceDirect Differentiation journal homepage: www.elsevier.com/locate/diff ☆ Development of the human penis and clitoris ⁎ ⁎⁎ Laurence Baskin , Joel Shen, Adriane Sinclair , Mei Cao, Xin Liu1, Ge Liu1, Dylan Isaacson, Maya Overland, Yi Li, Gerald R. Cunha UCSF, USA ARTICLE INFO ABSTRACT Keywords: The human penis and clitoris develop from the ambisexual genital tubercle. To compare and contrast the de- Development velopment of human penis and clitoris, we used macroscopic photography, optical projection tomography, light Human sheet microscopy, scanning electron microscopy, histology and immunohistochemistry. The human genital tu- Penis bercle differentiates into a penis under the influence of androgens forming a tubular urethra that develops by Clitoris canalization of the urethral plate to form a wide diamond-shaped urethral groove (opening zipper) whose edges Canalization and fusion (urethral folds) fuse in the midline (closing zipper). In contrast, in females, without the influence of androgens, the vestibular plate (homologue of the urethral plate) undergoes canalization to form a wide vestibular groove whose edges (vestibular folds) remain unfused, ultimately forming the labia minora defining the vaginal ves- tibule. The neurovascular anatomy is similar in both the developing human penis and clitoris and is the key to successful surgical reconstructions. 1. Introduction literature, in recent years we have recognized that the mouse is not the ideal model for normal human penile development and hypospadias for Male and female external genitalia play an essential role in human a host of reasons (Cunha et al., 2015; Liu et al., 2018b; Sinclair et al., reproduction, and disorders of structure and function of male and fe- 2016). -

Temporal and Spatial Dissection of Shh Signaling in Genital Tubercle Development Congxing Lin1, Yan Yin1, G
RESEARCH ARTICLE 3959 Development 136, 3959-3967 (2009) doi:10.1242/dev.039768 Temporal and spatial dissection of Shh signaling in genital tubercle development Congxing Lin1, Yan Yin1, G. Michael Veith1, Alexander V. Fisher1, Fanxin Long2,3 and Liang Ma1,3,* Genital tubercle (GT) initiation and outgrowth involve coordinated morphogenesis of surface ectoderm, cloacal mesoderm and hindgut endoderm. GT development appears to mirror that of the limb. Although Shh is essential for the development of both appendages, its role in GT development is much less clear than in the limb. Here, by removing Shh at different stages during GT development in mice, we demonstrate a continuous requirement for Shh in GT initiation and subsequent androgen-independent GT growth. Moreover, we investigated the Hh responsiveness of different tissue layers by removing or activating its signal transducer Smo with tissue-specific Cre lines, and established GT mesenchyme as the primary target tissue of Shh signaling. Lastly, we showed that Shh is required for the maintenance of the GT signaling center distal urethral epithelium (dUE). By restoring Wnt- Fgf8 signaling in Shh–/– cloacal endoderm genetically, we revealed that Shh relays its signal partly through the dUE, but regulates Hoxa13 and Hoxd13 expression independently of dUE signaling. Altogether, we propose that Shh plays a central role in GT development by simultaneously regulating patterning of the cloacal field and supporting an outgrowth signal. KEY WORDS: Shh, Genital tubercle, Cloaca, Hox, Mouse INTRODUCTION malformations are often accompanied by a persistent cloaca External genitalia (the penis in males and clitoris in females) are (Haraguchi et al., 2001; Haraguchi et al., 2007; Perriton et al., 2002; reproductive organs specialized for internal fertilization. -
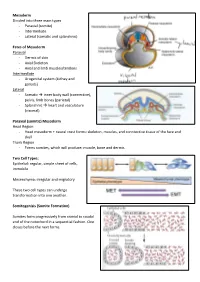
Mesoderm Divided Into Three Main Types - Paraxial (Somite) - Intermediate - Lateral (Somatic and Splanchnic)
Mesoderm Divided into three main types - Paraxial (somite) - Intermediate - Lateral (somatic and splanchnic) Fates of Mesoderm Paraxial - Dermis of skin - Axial Skeleton - Axial and limb muscles/tendons Intermediate - Urogenital system (kidney and gonads) Lateral - Somatic inner body wall (connective), pelvis, limb bones (parietal) - Splanchnic heart and vasculature (visceral) Paraxial (somitic) Mesoderm Head Region - Head mesoderm + neural crest forms: skeleton, muscles, and conntective tissue of the face and skull Trunk Region - Forms somites, which will produce: muscle, bone and dermis Two Cell Types: Epithelial: regular, simple sheet of cells, immobile Mesenchyma: irregular and migratory These two cell types can undergo transformation into one another. Somitogenisis (Somite Formation) Somites form progressively from cranial to caudal end of the notochord in a sequential fashion. One closes before the next forms. Somite Differentiation The somite splits into the epithelial dermamyotome (dermis/muscle) and the messenchymal sclerotome (skeletal). The somite is all paraxial mesoderm. Somite location determines the fate of its associates derma/myo/sclerotomes. Intermediate Mesoderm Urogenital system: - Kidneys - Gonads - Reproductive Duct Systems Runs alongside the paraxial mesoderm. Urogenital System Along mesonephric duct: - Pronephros, mesonephros, and metanephros - Pronephros fall away as gonad develops on ventral-medial side of mesonephros. - Metanephrogenic mesenchyme gives rise to kidney. The mesonephric duct will become the Wolffian duct forming at the nephric bud. The Mullerian duct forms via an invagination on the dorsal side of the nephric duct. The gonad will degenerate one of the two ducts depending on the hormones it produces. XX degenerates Wolffian duct – no testosterone, anti-Mullerian hormone (AMH) not produced, and Mullerian duct can develop in addition to female reproductive organs (ovaries, vagina) XY degenerates Mullerian duct – testosterone, AMH produced, Wolffian duct continues as male reproductive organs (testes, penis) develop. -

46/Xx Chromosome Constitution of a True Hermaphrodite
24 April 1965 S.A. TYDSKRIF VIR GENEESKUNDE 327 46/XX CHROMOSOME CONSTITUTION OF A TRUE HERMAPHRODITE S. H. FRJEDBERG, Department of Anatomy, University of the Witwarersrand Medical School, Johannesburg, AND E. E. ROSENBERG, Department of Physiology, FacullY of Medicine, University of Natal Medical School, Durban The influence of the Y-~hromosome in sex deyelopment of a normal-sized bicornuate uterus, through the inguinal is apparently considerable, since testes and a male pheno canal, into the labium majus. Here it was associated with a mass which was partially covered with a processus vaginalis type are observed in individuals who possess as many as and had the outward appearance of an ovary. This proved 5 X-chromosomes in addition to the Y.l Since a single histologically to be an ovotestis (Fig. 3). It contained numerous X-chromosome in the absence of a Y results in a female corpora lutea, a few corpora albicantia and seminiferous phenotype, it appears in fact that the Y -chromosome is a tubules with marked thickening and hyalinosis of the base ment membrane. The tubules were lined by Sertoli-like cells prerequisite for the development of human testicular and a few spermatogonia. There was no evidence of spermato tissue. The fact that the majority of true hermaphrodites genesis. The interstitial cells of Leydig were hyperplastic (Fig. (19 out of 25) have a normal female XX complex2 is thus 4). puzzling. It would seem that though the Y-chromosome The left gonad was cystic and haemorrhagic and proved histologically to consist only of ovarian tissue. The left has considerable influence in testicular differentiation, it uterine tube had undergone torsion. -

Renal Development
RENAL DEVELOPMENT Jon Barasch M.D., Ph.D. Telephone: 305-1890 e-mail: [email protected] SUGGESTED READING: Larsen, 3rd edition, pp 265 (first three paragraphs) - 266, 268-276 and figure 10-10 LEARNING OBJECTIVES: You should be able to: 1. Describe the three kidneys that are produced during development and know what happens to each one. 2. Explain what is meant by ‘reciprocal induction’ and why it poses problems in interpreting experiments in developing kidney. 3. Describe the stages of nephron formation from the renal vesicle. 4. Discuss the regulators of mesenchymal to epithelial transition in the intermediate mesoderm and metanephric mesenchyme and name three molecules mediating conversion. 5. Describe branching morphogenesis and name the three patterns in the developing metanephros. 6. Discuss three key important ligands and their receptors. 7. Discuss the classification of congenital renal abnormalities that are associated with urological abnormalities and the possible underlying mechanisms for their association. SUMMARY: The urogenital system derives from mesenchymal cells by a process of conversion to epithelia. The development of the kidney relies on three mechanisms of epithelial morphogenesis. 1. Some newborn epithelia migrate extensively (Wolffian duct), 2. some undergo branching morphogenesis (ureteric bud) and 3. some produce highly segmented tubules (nephrons). GLOSSARY: Angiotensin II: your favorite vasoconstrictor and regulator of proximal tubule reclamation of NaCl and water by receptor type 1. Receptor type-2 modulates cell growth and seems to play a role in congenital abnormalities. Arcade: a tubule of ureteric bud that induces a few nephrons simultaneously. The nephrons join to a common drainage called a connecting tubule that feeds into the ureteric’s collecting duct. -

Genital System
Development of Urogenital System Dr. Ammar Alhaaik Development of Urinary System Diagrams of transverse sections of the embryo - 20 days Intermediate mesoderm Intermediate mesoderm (including adjacent coelom mesothelium) forms a urogenital ridge, consisting of a laterally-positioned nephrogenic cord (that becomes kidneys & ureter) and a medially positioned gonadal ridge (for ovary/testis & female/male genital tract formation). Urinary & genital systems have a common embryonic origin; also, they share common ducts. Nephrogenic cord: develops into three sets of tubular nephric structures (from head to tail :)pronephros mesonephros metanephros) Pronephros— develops during the 4th weekin the cervical region of the intermediate mesoderm . It consists of (7-8) primitive tubules and a pronephric duct that grows caudally and terminates in the cloaca. The tubules soon degenerate, but the pronephric duct persists as the mesonephric duct. Mesonephros: — consists of (70-80) tubules induced to form by the mesonephric duct (former pronephric duct) — one end of each tubule surrounds a glomerulus (vascular proliferation produced by a branch of the dorsal aorta) — the other end of the tubule communicates with the mesonephric duct — eventually, the mesonephros degenerates, but the mesonephric duct becomes epididymis & ductus deferens and some tubules that become incorporated within the testis Metanephros: *becomes adult kidney & ureter of mammals, birds, and reptiles • originates in the pelvic region and moves cranially into the abdomen during embryonic differential growth * lobulated initially but becomes smooth in most species The metanephros originates from two sources: 1- a ureteric bud, which grows out of the mesonephric duct near the cloaca; the bud develops into the ureter, renal pelvis, and numerous collecting ducts; 2- metanephrogenic blastema, which is the caudal region of the nephrogenic cord; the mass forms nephrons NOTE • The pronephros is not functional.