Reproductionreview
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -
The Reproductive System
27 The Reproductive System PowerPoint® Lecture Presentations prepared by Steven Bassett Southeast Community College Lincoln, Nebraska © 2012 Pearson Education, Inc. Introduction • The reproductive system is designed to perpetuate the species • The male produces gametes called sperm cells • The female produces gametes called ova • The joining of a sperm cell and an ovum is fertilization • Fertilization results in the formation of a zygote © 2012 Pearson Education, Inc. Anatomy of the Male Reproductive System • Overview of the Male Reproductive System • Testis • Epididymis • Ductus deferens • Ejaculatory duct • Spongy urethra (penile urethra) • Seminal gland • Prostate gland • Bulbo-urethral gland © 2012 Pearson Education, Inc. Figure 27.1 The Male Reproductive System, Part I Pubic symphysis Ureter Urinary bladder Prostatic urethra Seminal gland Membranous urethra Rectum Corpus cavernosum Prostate gland Corpus spongiosum Spongy urethra Ejaculatory duct Ductus deferens Penis Bulbo-urethral gland Epididymis Anus Testis External urethral orifice Scrotum Sigmoid colon (cut) Rectum Internal urethral orifice Rectus abdominis Prostatic urethra Urinary bladder Prostate gland Pubic symphysis Bristle within ejaculatory duct Membranous urethra Penis Spongy urethra Spongy urethra within corpus spongiosum Bulbospongiosus muscle Corpus cavernosum Ductus deferens Epididymis Scrotum Testis © 2012 Pearson Education, Inc. Anatomy of the Male Reproductive System • The Testes • Testes hang inside a pouch called the scrotum, which is on the outside of the body -

Clinical Pelvic Anatomy
SECTION ONE • Fundamentals 1 Clinical pelvic anatomy Introduction 1 Anatomical points for obstetric analgesia 3 Obstetric anatomy 1 Gynaecological anatomy 5 The pelvic organs during pregnancy 1 Anatomy of the lower urinary tract 13 the necks of the femora tends to compress the pelvis Introduction from the sides, reducing the transverse diameters of this part of the pelvis (Fig. 1.1). At an intermediate level, opposite A thorough understanding of pelvic anatomy is essential for the third segment of the sacrum, the canal retains a circular clinical practice. Not only does it facilitate an understanding cross-section. With this picture in mind, the ‘average’ of the process of labour, it also allows an appreciation of diameters of the pelvis at brim, cavity, and outlet levels can the mechanisms of sexual function and reproduction, and be readily understood (Table 1.1). establishes a background to the understanding of gynae- The distortions from a circular cross-section, however, cological pathology. Congenital abnormalities are discussed are very modest. If, in circumstances of malnutrition or in Chapter 3. metabolic bone disease, the consolidation of bone is impaired, more gross distortion of the pelvic shape is liable to occur, and labour is likely to involve mechanical difficulty. Obstetric anatomy This is termed cephalopelvic disproportion. The changing cross-sectional shape of the true pelvis at different levels The bony pelvis – transverse oval at the brim and anteroposterior oval at the outlet – usually determines a fundamental feature of The girdle of bones formed by the sacrum and the two labour, i.e. that the ovoid fetal head enters the brim with its innominate bones has several important functions (Fig. -

Cranial Cavitry
Embryology Endo, Energy, and Repro 2017-2018 EMBRYOLOGY OF THE REPRODUCTIVE SYSTEM Janine Prange-Kiel, Ph.D. Office: L1.106, Phone: 83117 Email: [email protected] LEARNING OBJECTIVES: • Name the structures in kidney development that contribute to the development of the reproductive organs. • Predict how the presence or absence of the Y chromosome and the expression of the SRY gene would influence the development of the gonads. • Predict how the presence or absence of testosterone, dihydrotestosterone, and anit- Mullerian hormone would influence the development of the genital ducts and indifferent primordia of the external genitalia. I. Introduction In general, the function of the genital (reproductive) system in males and females is the formation, nurture, and transport of germ cells. In females, an additional function is to provide the proper milieu for the fetal development after conception. Like the urinary system, the genital system derives from intermediate mesoderm. The development of these two systems is tightly interwoven as structures that develop as parts of the urinary system gain function in the genital system. In the adult, the sexual organs differ between males and females. The early genital system, however, is similar in both sexes, and the sexual differentiation of this initially indifferent, bipotential system starts only in the seventh week of embryonic development. The details on how sexual differentiation is determined will be discussed below, but it is worth mentioning here that irregularities in this process result in disorders of sexual differentiation (DSDs). DSDs occur in approximately 1 in 4,500 live births and will be discussed in a separate lecture. -

Terminologia Embryologica
Terminologia Embryologica МЕЖДУНАРОДНЫЕ ТЕРМИНЫ ПО ЭМБРИОЛОГИИ ЧЕЛОВЕКА С ОФИЦИАЛЬНЫМ СПИСКОМ РУССКИХ ЭКВИВАЛЕНТОВ FEDERATIVE INTERNATIONAL PROGRAMME ON ANATOMICAL TERMINOLOGIES (FIPAT) РОССИЙСКАЯ ЭМБРИОЛОГИЧЕСКАЯ НОМЕНКЛАТУРНАЯ КОМИССИЯ Под редакцией акад. РАН Л.Л. Колесникова, проф. Н.Н. Шевлюка, проф. Л.М. Ерофеевой 2014 VI СОДЕРЖАНИЕ 44 Facies Лицо Face 46 Systema digestorium Пищеварительная система Alimentary system 47 Cavitas oris Ротовая полость Oral cavity 51 Pharynx Глотка Pharynx 52 Canalis digestorius; Canalis Пищеварительный канал Alimentary canal oesophagogastrointestinalis 52 Oesophagus Пищевод Oesophagus▲ 53 Gaster Желудок Stomach 54 Duodenum Двенадцатиперстная кишка Duodenum 55 Ansa umbilicalis intestini Пупочная кишечная петля Midgut loop; Umbilical intestinal loop 56 Jejunum et Ileum Тощая и подвздошная кишка Jejunum and Ileum 56 Intestinum crassum Толстая кишка Large intestine 58 Canalis analis Анальный канал Anal canal 58 Urenteron; Pars postcloacalis Постклоакальная часть кишки Postcloacal gut; intestini Tailgut; Endgut 59 Hepar Печень Liver 60 Ductus choledochus; Ductus Жёлчный проток Bile duct biliaris 61 Vesica biliaris et ductus Жёлчный пузырь и пузырный про- Gallbladder and cystic cysticus ток duct 61 Pancreas Поджелудочная железа Pancreas 63 Systema respiratorium Дыхательная система Respiratory system 63 Nasus Нос Nose 64 Pharynx Глотка, зев Pharynx 64 Formatio arboris respiratoriae Формирование дыхательной Formation of системы (бронхиального дерева) respiratory tree 67 Systema urinarium Мочевая система Urinary -

Short Communication Clinical and Pathological
Reprod Dom Anim 45, 368–372 (2010); doi: 10.1111/j.1439-0531.2009.01495.x ISSN 0936-6768 Short Communication Clinical and Pathological Findings of a Sac-like Formation in the Tunica Vaginalis of a Nelore (Bos indicus) Bull J Chaco´n1 and A Berrocal2,* 1Section of Andrology, Research Program on Applied Animal Andrology; 2Department of Pathology, School of Veterinary Medicine, Universidad, Nacional (UNA), Heredia, Costa Rica Contents and abnormalities in the ejaculate compared with A seven-month-old purebred Nelore calf was diagnosed with a normal bulls (Chaco´n et al. 1999; Chaco´n 2001). bilateral finger-shaped swelling although more prominent at Regarding the tunica vaginalis in the bull, abnormal- the left side of the scrotum, located longitudinal and parallel to ities reported in this serosa are mainly limited to epididymis corpus. The finding was present from 7 months of conditions such as hydrocele and adherences within age up to castration (performed at 25 months of age). Scrotal tunica layers secondary to orchitis. Blom and Christen- circumference, testicular and epididymis consistency and sen (1958) described also the presence of cyst like symmetry as well as seminal quality were normal during the formations (paradidymis), in the funiculus spermaticus follow-up period. The ultrasonographic appearance of the scrotal wall, pampiniform plexus, gonad and epididymis was (mesorchium), presumable derived from remnants of the normal. However, an anechoic region surrounded by a wall mesonephric duct. It is unknown that sac-like forma- forming a sac-like structure with a blind end at its dorsal pole tions in the tunica vaginalis of the bull or any other was seen on the swelling area. -

Reproductionreview
REPRODUCTIONREVIEW Cryptorchidism in common eutherian mammals R P Amann and D N R Veeramachaneni Animal Reproduction and Biotechnology Laboratory, Colorado State University, Fort Collins, Colorado 80523-1683, USA Correspondence should be addressed to R P Amann; Email: [email protected] Abstract Cryptorchidism is failure of one or both testes to descend into the scrotum. Primary fault lies in the testis. We provide a unifying cross-species interpretation of testis descent and urge the use of precise terminology. After differentiation, a testis is relocated to the scrotum in three sequential phases: abdominal translocation, holding a testis near the internal inguinal ring as the abdominal cavity expands away, along with slight downward migration; transinguinal migration, moving a cauda epididymidis and testis through the abdominal wall; and inguinoscrotal migration, moving a s.c. cauda epididymidis and testis to the bottom of the scrotum. The gubernaculum enlarges under stimulation of insulin-like peptide 3, to anchor the testis in place during gradual abdominal translocation. Concurrently, testosterone masculinizes the genitofemoral nerve. Cylindrical downward growth of the peritoneal lining into the gubernaculum forms the vaginal process, cremaster muscle(s) develop within the gubernaculum, and the cranial suspensory ligament regresses (testosterone not obligatory for latter). Transinguinal migration of a testis is rapid, apparently mediated by intra-abdominal pressure. Testosterone is not obligatory for correct inguinoscrotal migration of testes. However, normally testosterone stimulates growth of the vaginal process, secretion of calcitonin gene-related peptide by the genitofemoral nerve to provide directional guidance to the gubernaculum, and then regression of the gubernaculum and constriction of the inguinal canal. Cryptorchidism is more common in companion animals, pigs, or humans (2–12%) than in cattle or sheep (%1%). -

Imperforate Anus and Cloacal Malformations Marc A
C H A P T E R 3 5 Imperforate Anus and Cloacal Malformations Marc A. Levitt • Alberto Peña ‘Imperforate anus’ has been a well-known condition since component but were left with a persistent urogenital antiquity.1–3 For many centuries, physicians, as well as sinus.21,23 Additionally, most rectovestibular fistulas were individuals who practiced medicine, have tried to help erroneously called ‘rectovaginal fistula’.21 A rectoblad- these children by creating an orifice in the perineum. derneck fistula in males is the only true supralevator Many patients survived, most likely because they suffered malformation and occurs in about 10%.18 As it is the only from a type of defect that is now recognized as ‘low.’ malformation in males in which the rectum is unreach- Those with a ‘high’ defect did not survive. In 1835, able through a posterior sagittal incision, it requires an Amussat was the first to suture the rectal wall to the skin abdominal approach (via laparoscopy or a laparotomy) in edges which was the first actual anoplasty.2 Stephens addition to the perineal approach. made a significant contribution by performing the first Anorectal malformations represent a wide spectrum of anatomic studies in human specimens. In 1953, he pro- defects. The terms ‘low,’ ‘intermediate,’ and ‘high’ are arbi- posed an initial sacral approach followed by an abdomi- trary and not useful in current therapeutic or prognostic noperineal operation, if needed.4 The purpose of the terminology. A therapeutic and prognostically oriented sacral stage of this procedure was to preserve the pub- classification is depicted in Box 35-1.24 orectalis sling, considered a key factor in maintaining fecal incontinence. -

ANA214: Systemic Embryology
ANA214: Systemic Embryology ISHOLA, Azeez Olakune [email protected] Anatomy Department, College of Medicine and Health Sciences Outline • Organogenesis foundation • Urogenital system • Respiratory System • Kidney • Larynx • Ureter • Trachea & Bronchi • Urinary bladder • Lungs • Male urethra • Female urethra • Cardiovascular System • Prostate • Heart • Uterus and uterine tubes • Blood vessels • Vagina • Fetal Circulation • External genitalia • Changes in Circulation at Birth • Testes • Gastrointestinal System • Ovary • Mouth • Nervous System • Pharynx • Neurulation • GI Tract • Neural crests • Liver, Spleen, Pancreas Segmentation of Mesoderm • Start by 17th day • Under the influence of notochord • Cells close to midline proliferate – PARAXIAL MESODERM • Lateral cells remain thin – LATERAL PLATE MESODERM • Somatic/Parietal mesoderm – close to amnion • Visceral/Splanchnic mesoderm – close to yolk sac • Intermediate mesoderm connects paraxial and lateral mesoderm Paraxial Mesoderm • Paraxial mesoderm organized into segments – SOMITOMERES • Occurs in craniocaudal sequence and start from occipital region • 1st developed by day 20 (3 pairs per day) – 5th week • Gives axial skeleton Intermediate Mesoderm • Differentiate into Urogenital structures • Pronephros, mesonephros Lateral Plate Mesoderm • Parietal mesoderm + ectoderm = lateral body wall folds • Dermis of skin • Bones + CT of limb + sternum • Visceral Mesoderm + endoderm = wall of gut tube • Parietal mesoderm surrounding cavity = pleura, peritoneal and pericardial cavity • Blood & -

Early Vaginal Replacement in Cloacal Malformation
Pediatric Surgery International (2019) 35:263–269 https://doi.org/10.1007/s00383-018-4407-1 ORIGINAL ARTICLE Early vaginal replacement in cloacal malformation Shilpa Sharma1 · Devendra K. Gupta1 Accepted: 18 October 2018 / Published online: 30 October 2018 © Springer-Verlag GmbH Germany, part of Springer Nature 2018 Abstract Purpose We assessed the surgical outcome of cloacal malformation (CM) with emphasis on need and timing of vaginal replacement. Methods An ambispective study of CM was carried out including prospective cases from April 2014 to December 2017 and retrospective cases that came for routine follow-up. Early vaginal replacement was defined as that done at time of bowel pull through. Surgical procedures and associated complications were noted. The current state of urinary continence, faecal continence and renal functions was assessed. Results 18 patients with CM were studied with median age at presentation of 5 days (1 day–4 years). 18;3;2 babies underwent colostomy; vaginostomy; vesicostomy. All patients underwent posterior sagittal anorectovaginourethroplasty (PSARVUP)/ Pull through at a median age of 13 (4–46) months. Ten patients had long common channel length (> 3 cm). Six patients underwent early vaginal replacement at a median age of 14 (7–25) months with ileum; sigmoid colon; vaginal switch; hemirectum in 2;2;1;1. Three with long common channel who underwent only PSARVUP had inadequate introitus at puberty. Complications included anal mucosal prolapse, urethrovaginal fistula, perineal wound dehiscence, pyometrocolpos, blad- der injury and pelvic abscess. Persistent vesicoureteric reflux remained in 8. 5;2 patients had urinary; faecal incontinence. 2 patients of uterus didelphys are having menorrhagia. -
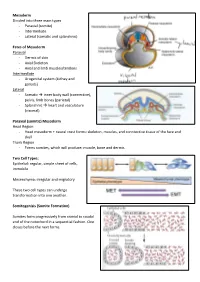
Mesoderm Divided Into Three Main Types - Paraxial (Somite) - Intermediate - Lateral (Somatic and Splanchnic)
Mesoderm Divided into three main types - Paraxial (somite) - Intermediate - Lateral (somatic and splanchnic) Fates of Mesoderm Paraxial - Dermis of skin - Axial Skeleton - Axial and limb muscles/tendons Intermediate - Urogenital system (kidney and gonads) Lateral - Somatic inner body wall (connective), pelvis, limb bones (parietal) - Splanchnic heart and vasculature (visceral) Paraxial (somitic) Mesoderm Head Region - Head mesoderm + neural crest forms: skeleton, muscles, and conntective tissue of the face and skull Trunk Region - Forms somites, which will produce: muscle, bone and dermis Two Cell Types: Epithelial: regular, simple sheet of cells, immobile Mesenchyma: irregular and migratory These two cell types can undergo transformation into one another. Somitogenisis (Somite Formation) Somites form progressively from cranial to caudal end of the notochord in a sequential fashion. One closes before the next forms. Somite Differentiation The somite splits into the epithelial dermamyotome (dermis/muscle) and the messenchymal sclerotome (skeletal). The somite is all paraxial mesoderm. Somite location determines the fate of its associates derma/myo/sclerotomes. Intermediate Mesoderm Urogenital system: - Kidneys - Gonads - Reproductive Duct Systems Runs alongside the paraxial mesoderm. Urogenital System Along mesonephric duct: - Pronephros, mesonephros, and metanephros - Pronephros fall away as gonad develops on ventral-medial side of mesonephros. - Metanephrogenic mesenchyme gives rise to kidney. The mesonephric duct will become the Wolffian duct forming at the nephric bud. The Mullerian duct forms via an invagination on the dorsal side of the nephric duct. The gonad will degenerate one of the two ducts depending on the hormones it produces. XX degenerates Wolffian duct – no testosterone, anti-Mullerian hormone (AMH) not produced, and Mullerian duct can develop in addition to female reproductive organs (ovaries, vagina) XY degenerates Mullerian duct – testosterone, AMH produced, Wolffian duct continues as male reproductive organs (testes, penis) develop. -

Genital System
Development of Urogenital System Dr. Ammar Alhaaik Development of Urinary System Diagrams of transverse sections of the embryo - 20 days Intermediate mesoderm Intermediate mesoderm (including adjacent coelom mesothelium) forms a urogenital ridge, consisting of a laterally-positioned nephrogenic cord (that becomes kidneys & ureter) and a medially positioned gonadal ridge (for ovary/testis & female/male genital tract formation). Urinary & genital systems have a common embryonic origin; also, they share common ducts. Nephrogenic cord: develops into three sets of tubular nephric structures (from head to tail :)pronephros mesonephros metanephros) Pronephros— develops during the 4th weekin the cervical region of the intermediate mesoderm . It consists of (7-8) primitive tubules and a pronephric duct that grows caudally and terminates in the cloaca. The tubules soon degenerate, but the pronephric duct persists as the mesonephric duct. Mesonephros: — consists of (70-80) tubules induced to form by the mesonephric duct (former pronephric duct) — one end of each tubule surrounds a glomerulus (vascular proliferation produced by a branch of the dorsal aorta) — the other end of the tubule communicates with the mesonephric duct — eventually, the mesonephros degenerates, but the mesonephric duct becomes epididymis & ductus deferens and some tubules that become incorporated within the testis Metanephros: *becomes adult kidney & ureter of mammals, birds, and reptiles • originates in the pelvic region and moves cranially into the abdomen during embryonic differential growth * lobulated initially but becomes smooth in most species The metanephros originates from two sources: 1- a ureteric bud, which grows out of the mesonephric duct near the cloaca; the bud develops into the ureter, renal pelvis, and numerous collecting ducts; 2- metanephrogenic blastema, which is the caudal region of the nephrogenic cord; the mass forms nephrons NOTE • The pronephros is not functional.