Systematics of the Pristimantis Phoxocephalus Species Complex (Anura, Craugastoridae) in Ecuador
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Catalogue of the Amphibians of Venezuela: Illustrated and Annotated Species List, Distribution, and Conservation 1,2César L
Mannophryne vulcano, Male carrying tadpoles. El Ávila (Parque Nacional Guairarepano), Distrito Federal. Photo: Jose Vieira. We want to dedicate this work to some outstanding individuals who encouraged us, directly or indirectly, and are no longer with us. They were colleagues and close friends, and their friendship will remain for years to come. César Molina Rodríguez (1960–2015) Erik Arrieta Márquez (1978–2008) Jose Ayarzagüena Sanz (1952–2011) Saúl Gutiérrez Eljuri (1960–2012) Juan Rivero (1923–2014) Luis Scott (1948–2011) Marco Natera Mumaw (1972–2010) Official journal website: Amphibian & Reptile Conservation amphibian-reptile-conservation.org 13(1) [Special Section]: 1–198 (e180). Catalogue of the amphibians of Venezuela: Illustrated and annotated species list, distribution, and conservation 1,2César L. Barrio-Amorós, 3,4Fernando J. M. Rojas-Runjaic, and 5J. Celsa Señaris 1Fundación AndígenA, Apartado Postal 210, Mérida, VENEZUELA 2Current address: Doc Frog Expeditions, Uvita de Osa, COSTA RICA 3Fundación La Salle de Ciencias Naturales, Museo de Historia Natural La Salle, Apartado Postal 1930, Caracas 1010-A, VENEZUELA 4Current address: Pontifícia Universidade Católica do Río Grande do Sul (PUCRS), Laboratório de Sistemática de Vertebrados, Av. Ipiranga 6681, Porto Alegre, RS 90619–900, BRAZIL 5Instituto Venezolano de Investigaciones Científicas, Altos de Pipe, apartado 20632, Caracas 1020, VENEZUELA Abstract.—Presented is an annotated checklist of the amphibians of Venezuela, current as of December 2018. The last comprehensive list (Barrio-Amorós 2009c) included a total of 333 species, while the current catalogue lists 387 species (370 anurans, 10 caecilians, and seven salamanders), including 28 species not yet described or properly identified. Fifty species and four genera are added to the previous list, 25 species are deleted, and 47 experienced nomenclatural changes. -

Species Diversity and Conservation Status of Amphibians in Madre De Dios, Southern Peru
Herpetological Conservation and Biology 4(1):14-29 Submitted: 18 December 2007; Accepted: 4 August 2008 SPECIES DIVERSITY AND CONSERVATION STATUS OF AMPHIBIANS IN MADRE DE DIOS, SOUTHERN PERU 1,2 3 4,5 RUDOLF VON MAY , KAREN SIU-TING , JENNIFER M. JACOBS , MARGARITA MEDINA- 3 6 3,7 1 MÜLLER , GIUSEPPE GAGLIARDI , LILY O. RODRÍGUEZ , AND MAUREEN A. DONNELLY 1 Department of Biological Sciences, Florida International University, 11200 SW 8th Street, OE-167, Miami, Florida 33199, USA 2 Corresponding author, e-mail: [email protected] 3 Departamento de Herpetología, Museo de Historia Natural de la Universidad Nacional Mayor de San Marcos, Avenida Arenales 1256, Lima 11, Perú 4 Department of Biology, San Francisco State University, 1600 Holloway Avenue, San Francisco, California 94132, USA 5 Department of Entomology, California Academy of Sciences, 55 Music Concourse Drive, San Francisco, California 94118, USA 6 Departamento de Herpetología, Museo de Zoología de la Universidad Nacional de la Amazonía Peruana, Pebas 5ta cuadra, Iquitos, Perú 7 Programa de Desarrollo Rural Sostenible, Cooperación Técnica Alemana – GTZ, Calle Diecisiete 355, Lima 27, Perú ABSTRACT.—This study focuses on amphibian species diversity in the lowland Amazonian rainforest of southern Peru, and on the importance of protected and non-protected areas for maintaining amphibian assemblages in this region. We compared species lists from nine sites in the Madre de Dios region, five of which are in nationally recognized protected areas and four are outside the country’s protected area system. Los Amigos, occurring outside the protected area system, is the most species-rich locality included in our comparison. -

The Most Frog-Diverse Place in Middle America, with Notes on The
Offcial journal website: Amphibian & Reptile Conservation amphibian-reptile-conservation.org 13(2) [Special Section]: 304–322 (e215). The most frog-diverse place in Middle America, with notes on the conservation status of eight threatened species of amphibians 1,2,*José Andrés Salazar-Zúñiga, 1,2,3Wagner Chaves-Acuña, 2Gerardo Chaves, 1Alejandro Acuña, 1,2Juan Ignacio Abarca-Odio, 1,4Javier Lobon-Rovira, 1,2Edwin Gómez-Méndez, 1,2Ana Cecilia Gutiérrez-Vannucchi, and 2Federico Bolaños 1Veragua Foundation for Rainforest Research, Limón, COSTA RICA 2Escuela de Biología, Universidad de Costa Rica, San Pedro, 11501-2060 San José, COSTA RICA 3División Herpetología, Museo Argentino de Ciencias Naturales ‘‘Bernardino Rivadavia’’-CONICET, C1405DJR, Buenos Aires, ARGENTINA 4CIBIO Research Centre in Biodiversity and Genetic Resources, InBIO, Universidade do Porto, Campus Agrário de Vairão, Rua Padre Armando Quintas 7, 4485-661 Vairão, Vila do Conde, PORTUGAL Abstract.—Regarding amphibians, Costa Rica exhibits the greatest species richness per unit area in Middle America, with a total of 215 species reported to date. However, this number is likely an underestimate due to the presence of many unexplored areas that are diffcult to access. Between 2012 and 2017, a monitoring survey of amphibians was conducted in the Central Caribbean of Costa Rica, on the northern edge of the Matama mountains in the Talamanca mountain range, to study the distribution patterns and natural history of species across this region, particularly those considered as endangered by the International Union for Conservation of Nature. The results show the highest amphibian species richness among Middle America lowland evergreen forests, with a notable anuran representation of 64 species. -

4. DUELLMAN, WE & E. LEHR (2009). Terrestrial-Breeding Frogs
PUBLICATIONS (133 in total) BOOKS & BOOK CONTRIBUTIONS (peer-reviewed*) 4. DUELLMAN, W. E. & E. LEHR (2009). Terrestrial-Breeding Frogs (Strabomantidae) in Peru. Natur- und Tier-Verlag, Naturwissenschaft, Münster, Germany, 382 pp. 3. LEHR, E. (2002). Amphibien und Reptilien in Peru: Die Herpetofauna entlang des 10. Breitengrades von Peru: Arterfassung, Taxonomie, ökologische Bemerkungen und biogeographische Beziehungen. Dissertation, Natur- und Tier-Verlag, Naturwissenschaft, Münster, Germany, 208 pp. 2. *BURKE, L. R., L. S. FORD, E. LEHR, S. MOCKFORD, P. C. H. PRITCHARD, J. P. O. ROSADO, D. M. SENNEKE, AND B. L. STUART (2007). Non-Standard Sources in a Standardized World: Responsible Practice and Ethics of Acquiring Turtle Specimens for Scientific Use, pp. 142– 146. In: SHAFFER, H.B., N. N. FITZSIMMONS, A. GEORGES & A. G.J. RHODIN (eds., 2007): Defining Turtle Diversity: Proceedings of a Workshop on Genetics, Ethics, and Taxonomy of Freshwater Turtles and Tortoises. Chelonian Research Monograph 4:1– 200. 1. *LEHR, E. (2005). The Telmatobius and Batrachophrynus (Anura: Leptodactylidae) species of Peru, pp. 39–64. In: E. O. LAVILLA & I. DE LA RIVA, (eds.), Studies on the Andean Frogs of the Genera Telmatobius and Batrachophrynus, Asociación Herpetológica Española, Monografías de Herpetología 7:1–349. SCIENTIFIC ARTICLES (peer-reviewed) 91. CATENAZZI, A., E. LEHR & R. VON MAY (2014): The amphibians and reptiles of Manu National Park and its buffer zone, Amazon basin and eastern slopes of the Andes, Peru. Biota Neotropica 13(4): 269–283. 90. MORAVEC, J., LEHR E., CUSI, J. C., CÓRDOVA, J. H. & V. Gvoždík (2014): A new species of the Rhinella margaritifera species group (Anura, Bufonidae) from the montane forest of the Selva Central, Peru. -

A Rapid Biological Assessment of the Upper Palumeu River Watershed (Grensgebergte and Kasikasima) of Southeastern Suriname
Rapid Assessment Program A Rapid Biological Assessment of the Upper Palumeu River Watershed (Grensgebergte and Kasikasima) of Southeastern Suriname Editors: Leeanne E. Alonso and Trond H. Larsen 67 CONSERVATION INTERNATIONAL - SURINAME CONSERVATION INTERNATIONAL GLOBAL WILDLIFE CONSERVATION ANTON DE KOM UNIVERSITY OF SURINAME THE SURINAME FOREST SERVICE (LBB) NATURE CONSERVATION DIVISION (NB) FOUNDATION FOR FOREST MANAGEMENT AND PRODUCTION CONTROL (SBB) SURINAME CONSERVATION FOUNDATION THE HARBERS FAMILY FOUNDATION Rapid Assessment Program A Rapid Biological Assessment of the Upper Palumeu River Watershed RAP (Grensgebergte and Kasikasima) of Southeastern Suriname Bulletin of Biological Assessment 67 Editors: Leeanne E. Alonso and Trond H. Larsen CONSERVATION INTERNATIONAL - SURINAME CONSERVATION INTERNATIONAL GLOBAL WILDLIFE CONSERVATION ANTON DE KOM UNIVERSITY OF SURINAME THE SURINAME FOREST SERVICE (LBB) NATURE CONSERVATION DIVISION (NB) FOUNDATION FOR FOREST MANAGEMENT AND PRODUCTION CONTROL (SBB) SURINAME CONSERVATION FOUNDATION THE HARBERS FAMILY FOUNDATION The RAP Bulletin of Biological Assessment is published by: Conservation International 2011 Crystal Drive, Suite 500 Arlington, VA USA 22202 Tel : +1 703-341-2400 www.conservation.org Cover photos: The RAP team surveyed the Grensgebergte Mountains and Upper Palumeu Watershed, as well as the Middle Palumeu River and Kasikasima Mountains visible here. Freshwater resources originating here are vital for all of Suriname. (T. Larsen) Glass frogs (Hyalinobatrachium cf. taylori) lay their -

Anura: Craugastoridae) with the Description of a New Species from Colombia
Acta Herpetologica 11(1): 31-45, 2016 DOI: 10.13128/Acta_Herpetol-16434 Molecular phylogenetics of the Pristimantis lacrimosus species group (Anura: Craugastoridae) with the description of a new species from Colombia Mauricio Rivera-Correa, Juan M. Daza* Grupo Herpetológico de Antioquia, Instituto de Biología, Universidad de Antioquia, Calle 67 # 53–108, Bloque 7–121, A.A. 1226, Medellín, Colombia. *Correspondig author. E-mail: [email protected] Submitted on 2015, 17th July; revised on 2015, 11thNovember; accepted on 2015, 16th November Editor: Adriana Bellati Abstract. The Pristimantis lacrimosus species group, with 24 species distributed in the Neotropics, is a group of arbo- real frogs commonly inhabiting bromeliads. Previous studies have claimed the group to be monophyletic but few spe- cies have been included in phylogenetic analyses. In this paper, we included five additional species from the northern Andes in Colombia and tested the monophyly of this phenetic group using genetic data under a Bayesian approach. Our results show that the P. lacrimosus group represents two distant and unrelated clades. Clade “A” is endemic to Colombia while Clade “B” encompasses species distributed in Central America, Ecuador and Peru. For the first time, we reveal the phylogenetic position of P. boulengeri and a new species is described. The new taxon is most closely related to P. brevifrons from southwestern Colombia with a genetic distance of 4.3% for 16S and 10.6% for COI. Our results suggest, one more time, that morphological similarity among species in the most diverse vertebrate genus not necessarily agree with its evolutionary history and that more effort in alpha taxonomy needs to be done in order to understand the tremendous radiation of this lineage in the Neotropics. -

Piemontano Oriental
guía dinámica de los anfibios del bosque piemontano oriental santiago ron coordinador editorial Lista de especies Número de especies: 134 Anura Hemiphractidae Gastrotheca testudinea, Rana marsupial de Jimenez de la Espada Gastrotheca weinlandii, Rana marsupial de Weinland Gastrotheca andaquiensis, Rana marsupial de Andaqui Hemiphractus proboscideus, Rana de cabeza triangular de Sumaco Hemiphractus scutatus, Rana de cabeza triangular cornuda incubadora Hemiphractus bubalus, Rana de cabeza triangular de Ecuador Hemiphractus helioi, Rana de cabeza triangular del Cuzco Bufonidae Atelopus boulengeri, Jambato de Boulenger Atelopus planispina, Jambato de planispina Atelopus spumarius, Jambato amazónico Atelopus palmatus, Jambato de Andersson Rhaebo ecuadorensis, Sapo gigante ecuatoriano Rhinella marina, Sapo de la caña Rhinella festae, Sapo del Valle de Santiago Rhinella ceratophrys, Sapo cornudo termitero Rhinella margaritifera, Sapo común sudamericano Rhinella dapsilis, Sapo orejón Rhinella poeppigii, Sapo de Monobamba Amazophrynella minuta, Sapo diminuto de hojarasca Centrolenidae Centrolene charapita, Cochranella resplendens, Rana de cristal resplandeciente Hyalinobatrachium pellucidum, Rana de cristal fantasma Nymphargus cochranae, Rana de cristal de Cochran Nymphargus chancas, Rana de cristal del Perú Nymphargus mariae, Rana de cristal de María Espadarana durrellorum, Rana de cristal de Jambué Rulyrana flavopunctata, Rana de cristal de puntos amarillos Rulyrana mcdiarmidi, Rana de cristal del Río Jambue Teratohyla midas, Rana de cristal -

Shifts in the Diversity of an Amphibian Community from a Premontane Forest of San Ramón, Costa Rica Cambios En La Diversidad De
DOI 10.15517/RBT.V67I2SUPL.37240 Artículo Shifts in the diversity of an amphibian community from a premontane forest of San Ramón, Costa Rica Cambios en la diversidad de una comunidad de anfibios en un bosque premontano de San Ramón, Costa Rica Víctor J. Acosta-Chaves1, 2* Víctor Madrigal-Elizondo3 Gerardo Chaves4 Brayan Morera-Chacón5 Adrián García-Rodríguez 4, 6 Federico Bolaños 4 1 Carrera de Turismo Ecológico, Universidad de Costa Rica Sede Atlántico, Recinto de Paraíso, Cartago, Costa Rica; [email protected]* 2 Red Mesoamericana y del Caribe para la Conservación de Anfibios y Reptiles. 3 Red de Áreas Protegidas, Universidad de Costa Rica, Sede Rodrigo Facio, San Pedro, Costa Rica; [email protected] 4 Escuela de Biología, Universidad de Costa Rica, San Pedro, 11501-2060 San José, Costa Rica; [email protected], [email protected] 5 Instituto Internacional para la Conservación y Manejo de Vida Silvestre, Universidad Nacional, Heredia, Costa Rica; [email protected] 6 Departamento de Zoología, Instituto de Biología, Universidad Nacional Autónoma de México, Mexico City, Mexico; [email protected] * Correspondence Received 05-X-2018 Corrected 18-I-2019 Accepted 06-II-2019 Abstract Biological communities are experiencing rapid shifts of composition in Neotropical ecosystems due to several factors causing population declines. However, emerging evidence has provided insights on the adaptive potential of multiple species to respond to illnesses and environmental pressures. In Costa Rica, the decline of amphibian populations is a remarkable example of these changes. Here we provide evidence of variation in the amphibian richness of a premontane forest of San Ramón (Costa Rica) across a ~30 year period. -

Danyella Paiva Da Silva Estrutura Das Assembleias De Anfíbios E Répteis
UNIVERSIDADE FEDERAL DO ACRE PRÓ-REITORIA DE PESQUISA E PÓS-GRADUAÇÃO PROGRAMA DE PÓS-GRADUAÇÃO EM ECOLOGIA E MANEJO DE RECURSOS NATURAIS Danyella Paiva da Silva Estrutura das assembleias de anfíbios e répteis em áreas ripárias e não ripárias do Parque Estadual Chandless, Acre. Dissertação de Mestrado I UNIVERSIDADE FEDERAL DO ACRE PRÓ-REITORIA DE PESQUISA E PÓS-GRADUAÇÃO PROGRAMA DE PÓS-GRADUAÇÃO EM ECOLOGIA E MANEJO DE RECURSOS NATURAIS Estrutura das assembleias de anfíbios e répteis em áreas ripárias e não ripárias do Parque Estadual Chandless, Acre. Danyella Paiva da Silva Dissertação apresentada ao Programa de Pós-Graduação em Ecologia e Manejo de Recursos Naturais da Universidade Federal do Acre, como parte dos requisitos para a obtenção do título de Mestre em Ecologia e Manejo de Recursos Naturais. Rio Branco, Acre, 2015. Universidade Federal do Acre II Pró-Reitoria de Pesquisa e Pós-Graduação Programa de Pós-Graduação em Ecologia e Manejo de Recursos Naturais Estrutura das assembleias de anfíbios e répteis em áreas ripárias e não ripárias do Parque Estadual Chandless, Acre. Danyella Paiva da Silva BANCA EXAMINADORA ORIENTADOR III AGRADECIMENTOS Primeiramente agradeço ao meu orientador Dr. Moisés Barbosa de Souza pela oportunidade proporcionada e pelo apoio e confiança que me foi dada em executar tão importante projeto de pesquisa. Aos professores que fizeram parte da minha banca de qualificação, Dr. Armando Muniz Calouro, Dr. Elder Ferreira Morato e Dr. Marcos Silveira que contribuíram com seu valioso conhecimento nas sugestões para a realização deste trabalho, e também, ao professor Dr. Cleber Ibraim Salimon por também contribuir com sugestões valiosas no projeto. -
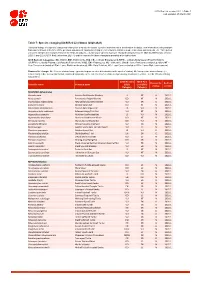
Table 7: Species Changing IUCN Red List Status (2020-2021)
IUCN Red List version 2021-1: Table 7 Last Updated: 25 March 2021 Table 7: Species changing IUCN Red List Status (2020-2021) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2020 (IUCN Red List version 2020-3) and 2021 (IUCN Red List version 2021-1) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered [CR(PE) - Critically Endangered (Possibly Extinct), CR(PEW) - Critically Endangered (Possibly Extinct in the Wild)], EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.); E - Previous listing was an Error. IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2020) List (2021) change version Category -

Molecular Phylogenetics of Pristimantis (Anura: Strabomantidae) and the Origin and Diversification of Central American Species
Molecular Phylogenetics of Pristimantis (Anura: Strabomantidae) and the origin and diversification of Central American species Nelsy Rocío Pinto Sánchez Trabajo de grado presentado para optar al título de M aestría en Ciencias Biológicas Director Santiago Madriñán, Ph.D. Profesor Asociado del Departamento de Ciencias Biológicas Universidad de los Andes Codirector Andrew J. Cra wford, Ph.D. Investigador Instituto Smithsonian de Investigaciones Tropicales Bogotá D. C., Noviembre 2008 ABSTRACT Pristimantis (Anura: Strabomantidae) represents an exceptionally diverse group among Neotropical anurans, but the evolutionary relationships among subgeneric groups are poorly known. Using both original and published multilocus DNA sequence data, we developed a novel phylogenetic hypothesis for this genus. Ingroup sampling included 30.9% of the described species (265 individuals from 132 species, of which 156 individuals from 34 species are new data). Genetic data included three mitochondrial (COI, 12S, 16S) and two nuclear markers (Rag-1 and Tyr) for a total of ~4279 base pairs. Phylogenies were inferred using parsimony, maximum likelihood and Bayesian analyses of individual genes and combined data sets. The new phylogenetic hypothesis conflicts with most recognized taxonomic groupings. Within Pristimantis, the peruvianus group was the only group recovered, whereas the "conscipillatus”, “curtipes”, “devillei”, “frater”, “lacrimosus”, “myersi”, “orestes”, “surdus”, and “unistrigatus” were not supported as natural groups. The molecular phylogeny suggests that the colonization of Central America by South American Pristimantis involved perhaps 13 independent events. Our results suggest alternative interpretations of Pristimantis taxonomy, character evolution, and biogeography, topics that now demand more extensive evaluation in future studies. Keywords: Anura, Strabomantidae, Pristimantis, Terrarana, Colombia, Central America, Great American Biotic Interchange, M olecular phylogenetics. -

A New, Critically Endangered Species of Pristimantis
SALAMANDRA 57(1): 15–26 New endangered species of Pristimantis from Peru SALAMANDRA 15 February 2021 ISSN 0036–3375 German Journal of Herpetology A new, critically endangered species of Pristimantis (Amphibia: Anura: Strabomantidae) from a mining area in the Cordillera Occidental of northern Peru (Región Cajamarca) Edgar Lehr1,2, Shenyu Lyu3 & Alessandro Catenazzi4 1) Department of Biology, Illinois Wesleyan University, P.O. Box 2900, Bloomington, IL 61701, USA 2) Departamento de Herpetología, Museo de Historia Natural, Universidad Nacional Mayor de San Marcos, Av. Arenales 1256, Jesús María, Lima 15072, Peru 3) School of Biological Sciences, Georgia Institute of Technology, Atlanta, GA 30332, USA 4) Florida International University, Department of Biological Sciences, 11200 SW 8th Street, Miami, FL 33199, USA Corresponding author: Edgar Lehr, e-mail: [email protected] Manuscript received: 4 September 2020 Accepted: 26 November 2020 by Jörn Köhler Abstract. We describe a new species of Pristimantis from high Andean grasslands (jalca) at 3600 m above sea level in northern Peru (Región Cajamarca) based on morphological and molecular characters. The new species is known from four males and five females, which were found sheltering in the rosettes ofPuya fastuosa (Bromeliaceae). The phylogenetic analysis of a fragment of the mitochondrial 16S rRNA gene suggests that the new species is a sister taxon of Pristimantis simonsii. The new species differs from its congeners by having a black dorsum speckled with white flecks and a dark brown groin with white spots. Furthermore, adult males have a snout–vent length of 23.6–27.2 mm (n = 4), and adult females of 25.6–32.8 mm (n = 5).