Fibrillar Self-Assembly of a Chimeric Elastin-Resilin Inspired Engineered Polypeptide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hdl 105112.Pdf
PUBLISHED VERSION Rajkamal Balu, Robert Knott, Nathan P. Cowieson, Christopher M. Elvin, Anita J. Hill, Namita R. Choudhury, Naba K. Dutta Structural ensembles reveal intrinsic disorder for the multi-stimuli responsive bio-mimetic protein Rec1-resilin Scientific Reports, 2015; 5:10896-1-10896-12 This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Scientific Reports | 5:10896 | DOI: 10.1038/srep10896 12 Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ Originally published at: http://doi.org/10.1038/srep10896 PERMISSIONS http://creativecommons.org/licenses/by/4.0/ 27 June 2017 http://hdl.handle.net/2440/105112 www.nature.com/scientificreports OPEN Structural ensembles reveal intrinsic disorder for the multi- stimuli responsive bio-mimetic Received: 31 October 2014 Accepted: 21 April 2015 protein Rec1-resilin Published: 04 June 2015 Rajkamal Balu1, Robert Knott2, Nathan P. Cowieson3, Christopher M. Elvin4, Anita J. Hill5, Namita R. Choudhury1 & Naba K. Dutta1 Rec1-resilin is the first recombinant resilin-mimetic protein polymer, synthesized from exon-1 of the Drosophila melanogaster gene CG15920 that has demonstrated unusual multi-stimuli responsiveness in aqueous solution. Crosslinked hydrogels of Rec1-resilin have also displayed remarkable mechanical properties including near-perfect rubber-like elasticity. The structural basis of these extraordinary properties is not clearly understood. -

The Flying Insect Thoracic Cuticle Is Heterogenous in Structure and in Thickness-Dependent Modulus Gradation
bioRxiv preprint doi: https://doi.org/10.1101/2021.06.30.450643; this version posted July 1, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 The flying insect thoracic cuticle is heterogenous in structure and in thickness-dependent 2 modulus gradation 3 4 Cailin Caseya, Claire Yagerb, Mark Jankauskia, Chelsea Heverana 5 Montana State University 6 a Mechanical and Industrial Engineering 7 b Ecology 8 Corresponding Authors: Chelsea Heveran and Mark Jankauski 9 [email protected], [email protected] 10 Present/ permanent address 220 Roberts Hall; Bozeman MT 59717 11 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.06.30.450643; this version posted July 1, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 12 Abstract 13 The thorax is a specialized structure central to an insect’s ability to fly. In the thorax, 14 flight muscles are surrounded by a thin layer of cuticle. The structure, composition, and material 15 properties of this chitinous structure may influence the efficiency of the thorax in flight. 16 However, these properties, as well as their variation throughout anatomical regions of the thorax 17 or between insect taxa, are not known. In this work, we provide a multi-faceted assessment of 18 thorax cuticle for fliers with asynchronous (honey bee; Apis mellifera) and synchronous 19 (hawkmoth; Manduca sexta) muscles. We investigated cuticle structure using histology, material 20 composition through confocal laser scanning microscopy, and modulus gradation with 21 nanoindentation. -

Arthropod Grasping and Manipulation: a Literature Review
Arthropod Grasping and Manipulation A Literature Review Aaron M. Dollar Harvard BioRobotics Laboratory Technical Report Department of Engineering and Applied Sciences Harvard University April 5, 2001 www.biorobotics.harvard.edu Introduction The purpose of this review is to report on the existing literature on the subject of arthropod grasping and manipulation. In order to gain a proper understanding of the state of the knowledge in this rather broad topic, it is necessary and appropriate to take a step backwards and become familiar with the basics of entomology and arthropod physiology. Once these principles have been understood it will then be possible to proceed towards the more specific literature that has been published in the field. The structure of the review follows this strategy. General background information will be presented first, followed by successively more specific topics, and ending with a review of the refereed journal articles related to arthropod grasping and manipulation. Background The phylum Arthropoda is the largest of the phyla, and includes all animals that have an exoskeleton, a segmented body in series, and six or more jointed legs. There are nine classes within the phylum, five of which the average human is relatively familiar with – insects, arachnids, crustaceans, centipedes, and millipedes. Of all known species of animals on the planet, 82% are arthropods (c. 980,000 species)! And this number just reflects the known species. Estimates put the number of arthropod species remaining to be discovered and named at around 9-30 million, or 10-30 times more than are currently known. And this is just the number of species; the population of each is another matter altogether. -

Mechanism of Resilin Elasticity
ARTICLE Received 24 Oct 2011 | Accepted 11 Jul 2012 | Published 14 Aug 2012 DOI: 10.1038/ncomms2004 Mechanism of resilin elasticity Guokui Qin1,*, Xiao Hu1,*, Peggy Cebe2 & David L. Kaplan1 Resilin is critical in the flight and jumping systems of insects as a polymeric rubber-like protein with outstanding elasticity. However, insight into the underlying molecular mechanisms responsible for resilin elasticity remains undefined. Here we report the structure and function of resilin from Drosophila CG15920. A reversible beta-turn transition was identified in the peptide encoded by exon III and for full-length resilin during energy input and release, features that correlate to the rapid deformation of resilin during functions in vivo. Micellar structures and nanoporous patterns formed after beta-turn structures were present via changes in either the thermal or the mechanical inputs. A model is proposed to explain the super elasticity and energy conversion mechanisms of resilin, providing important insight into structure–function relationships for this protein. Furthermore, this model offers a view of elastomeric proteins in general where beta-turn-related structures serve as fundamental units of the structure and elasticity. 1 Department of Biomedical Engineering, Tufts University, Medford, Massachusetts 02155, USA. 2 Department of Physics and Astronomy, Tufts University, Medford, Massachusetts 02155, USA. *These authors contributed equally to this work. Correspondence and requests for materials should be addressed to D.L.K. (email: [email protected]). -
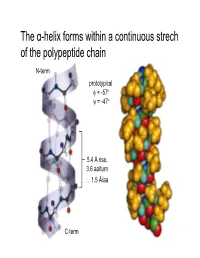
The Α-Helix Forms Within a Continuous Strech of the Polypeptide Chain
The α-helix forms within a continuous strech of the polypeptide chain N-term prototypical φ = -57 ° ψ = -47 ° 5.4 Å rise, 3.6 aa/turn ∴ 1.5 Å/aa C-term α-Helices have a dipole moment, due to unbonded and aligned N-H and C=O groups β-Sheets contain extended (β-strand) segments from separate regions of a protein prototypical φ = -139 °, ψ = +135 ° prototypical φ = -119 °, ψ = +113 ° (6.5Å repeat length in parallel sheet) Antiparallel β-sheets may be formed by closer regions of sequence than parallel Beta turn Figure 6-13 The stability of helices and sheets depends on their sequence of amino acids • Intrinsic propensity of an amino acid to adopt a helical or extended (strand) conformation The stability of helices and sheets depends on their sequence of amino acids • Intrinsic propensity of an amino acid to adopt a helical or extended (strand) conformation The stability of helices and sheets depends on their sequence of amino acids • Intrinsic propensity of an amino acid to adopt a helical or extended (strand) conformation • Interactions between adjacent R-groups – Ionic attraction or repulsion – Steric hindrance of adjacent bulky groups Helix wheel The stability of helices and sheets depends on their sequence of amino acids • Intrinsic propensity of an amino acid to adopt a helical or extended (strand) conformation • Interactions between adjacent R-groups – Ionic attraction or repulsion – Steric hindrance of adjacent bulky groups • Occurrence of proline and glycine • Interactions between ends of helix and aa R-groups His Glu N-term C-term -

Synthesis and Nmr Studies of a Β-Turn Mimetic Molecular
SYNTHESIS AND NMR STUDIES OF A β-TURN MIMETIC MOLECULAR TORSION BALANCE by Melissa Ann Liberatore B.S. Chemistry, Lehigh University, 2006 Submitted to the Graduate Faculty of the Kenneth P. Dietrich School of Arts and Sciences in partial fulfillment of the requirements for the degree of Doctor of Philosophy University of Pittsburgh 2012 UNIVERSITY OF PITTSBURGH DIETRICH SCHOOL OF ARTS AND SCIENCES This dissertation was presented by Melissa Ann Liberatore It was defended on July 23, 2012 and approved by Professor Dennis Curran, Department of Chemistry Professor Michael Trakselis, Department of Chemistry Professor Judith Klein-Seetharaman, Department of Structural Biology Dissertation Advisor: Professor Craig Wilcox, Department of Chemistry ii Copyright © by Melissa Ann Liberatore 2012 iii SYNTHESIS AND NMR STUDIES OF A β-TURN MIMETIC MOLECULAR TORSION BALANCE Melissa Ann Liberatore, PhD University of Pittsburgh, 2012 The attainment of precise measurements of the molecular forces that influence protein folding is important in order to further understand peptide dynamics and stability. A hybrid synthetic- natural peptide motif, combining an o,o,o’-trisubstituted biphenyl with an (ortho-tolyl)-amide, was synthesized in multiple formats and studied by NMR to probe the effects of amino acid substitutions on antiparallel beta-sheet configuration and stability. The potential of this “molecular torsion balance” as a beta-turn mimic was demonstrated by quantifying the rotational barriers about several axes. The free-energy rotational barrier of the aryl-aryl bond was found to be 35.7 kcal mol-1 at 418 K in hexanes. EXSY analysis was also used to measure barriers about -1 -1 the N-aryl (20.9 kcal mol at 343 K in toluene-d8) and N-CO bonds (17.2 kcal mol at 298 K in chloroform-d). -

Duke University Dissertation Template
Order and Disorder in Protein Biomaterial Design by Stefan Daniel Roberts Department of Biomedical Engineering Duke University Date:_______________________ Approved: ___________________________ Ashutosh Chilkoti, Supervisor ___________________________ Joel Collier ___________________________ Brenton Hoffman ___________________________ Terrence Oas ___________________________ Rohit Pappu Dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of Biomedical Engineering in the Graduate School of Duke University 2018 ABSTRACT Order and Disorder in Protein Biomaterial Design by Stefan Daniel Roberts Department of Biomedical Engineering Duke University Date:_______________________ Approved: ___________________________ Ashutosh Chilkoti, Supervisor ___________________________ Joel Collier ___________________________ Brenton Hoffman ___________________________ Terrence Oas ___________________________ Rohit Pappu An abstract of a dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of Biomedical Engineering in the Graduate School of Duke University 2018 Copyright by Stefan Daniel Roberts 2018 Abstract Crystalline and amorphous materials have been extensively studied for their interesting properties, but they comprise a very small portion of the total materials space. The properties of most materials are a consequence of the interactions between their ordered and disordered components. This phenomenon is especially -

Resilin Matrix Distribution, Variability and Function in Drosophila
Lerch et al. BMC Biology (2020) 18:195 https://doi.org/10.1186/s12915-020-00902-4 RESEARCH ARTICLE Open Access Resilin matrix distribution, variability and function in Drosophila Steven Lerch1,2,3, Renata Zuber1, Nicole Gehring2, Yiwen Wang2, Barbara Eckel1, Klaus-Dieter Klass3, Fritz-Olaf Lehmann4 and Bernard Moussian1,2,5* Abstract Background: Elasticity prevents fatigue of tissues that are extensively and repeatedly deformed. Resilin is a resilient and elastic extracellular protein matrix in joints and hinges of insects. For its mechanical properties, Resilin is extensively analysed and applied in biomaterial and biomedical sciences. However, there is only indirect evidence for Resilin distribution and function in an insect. Commonly, the presence of dityrosines that covalently link Resilin protein monomers (Pro-Resilin), which are responsible for its mechanical properties and fluoresce upon UV excitation, has been considered to reflect Resilin incidence. Results: Using a GFP-tagged Resilin version, we directly identify Resilin in pliable regions of the Drosophila body, some of which were not described before. Interestingly, the amounts of dityrosines are not proportional to the amounts of Resilin in different areas of the fly body, arguing that the mechanical properties of Resilin matrices vary according to their need. For a functional analysis of Resilin matrices, applying the RNA interference and Crispr/Cas9 techniques, we generated flies with reduced or eliminated Resilin function, respectively. We find that these flies are flightless but capable of locomotion and viable suggesting that other proteins may partially compensate for Resilin function. Indeed, localizations of the potentially elastic protein Cpr56F and Resilin occasionally coincide. Conclusions: Thus, Resilin-matrices are composite in the way that varying amounts of different elastic proteins and dityrosinylation define material properties. -

Production and Characterization of Recombinant Collagen-Binding Resilin Nanocomposite for Regenerative Medicine Applications
Regenerative Engineering and Translational Medicine (2019) 5:362–372 https://doi.org/10.1007/s40883-019-00092-8 Production and Characterization of Recombinant Collagen-Binding Resilin Nanocomposite for Regenerative Medicine Applications Paiyz E. Mikael 1 & Ranodhi Udangawa1,2 & Mirco Sorci1 & Brady Cress1,3 & Zvi Shtein4,5 & Georges Belfort1,3 & Oded Shoseyov4,5 & Jonathan S. Dordick1,3 & Robert J. Linhardt1,2,3 Received: 6 December 2018 /Accepted: 28 January 2019/Publishedonline:19 February 2019 # The Regenerative Engineering Society 2019 Abstract Development of mechanically stable and multifunctional biomaterials for sensing, repair, and regeneration applica- tions is of great importance. Herein, we investigate the potential of recombinant resilin-like (Res) nanocomposite elastomer as a template biomaterial for regenerative devices such as adhesive bandages or films, electrospun fibers, screws, sutures, and drug delivery vehicles.ExonI(Rec1)fromthenativeresilingeneofDrosophila (CG15920) was fused with collagen-binding domain (ColBD) from Clostridium histolyticum and expressed in Komagataella pastoris (formerly Pichia pastoris). The 100% binding of Resilin-ColBD (Res-ColBD) to collagen I was shown at a1:1ratiobymass.AtomicforcemicroscopyresultsinforcemodeshowabimodalprofilefortheColBD-binding interactions. Moreover, based on the force-volume map, Res-ColBD adhesion to collagen was statistically signifi- cantly higher than resilin without ColBD. Lay Summary Designing advanced biomaterials that will not only withstand the repetitive mechanical -

Pi-Pi Contacts Are an Overlooked Protein Feature Relevant to Phase
RESEARCH ARTICLE Pi-Pi contacts are an overlooked protein feature relevant to phase separation Robert McCoy Vernon1, Paul Andrew Chong1, Brian Tsang1,2, Tae Hun Kim1, Alaji Bah1†, Patrick Farber1‡, Hong Lin1, Julie Deborah Forman-Kay1,2* 1Program in Molecular Medicine, Hospital for Sick Children, Toronto, Canada; 2Department of Biochemistry, University of Toronto, Toronto, Canada Abstract Protein phase separation is implicated in formation of membraneless organelles, signaling puncta and the nuclear pore. Multivalent interactions of modular binding domains and their target motifs can drive phase separation. However, forces promoting the more common phase separation of intrinsically disordered regions are less understood, with suggested roles for multivalent cation-pi, pi-pi, and charge interactions and the hydrophobic effect. Known phase- separating proteins are enriched in pi-orbital containing residues and thus we analyzed pi- interactions in folded proteins. We found that pi-pi interactions involving non-aromatic groups are widespread, underestimated by force-fields used in structure calculations and correlated with solvation and lack of regular secondary structure, properties associated with disordered regions. We present a phase separation predictive algorithm based on pi interaction frequency, highlighting proteins involved in biomaterials and RNA processing. DOI: https://doi.org/10.7554/eLife.31486.001 *For correspondence: [email protected] Introduction † Present address: Department Protein phase separation has important implications for cellular organization and signaling of Biochemistry and Molecular (Mitrea and Kriwacki, 2016; Brangwynne et al., 2009; Su et al., 2016), RNA processing Biology, SUNY Upstate Medical University, New York, United (Sfakianos et al., 2016), biological materials (Yeo et al., 2011) and pathological aggregation States; ‡Zymeworks, Vancouver, (Taylor et al., 2016). -

Using Molecular Constraints and Unnatural Amino Acids to Manipulate and Interrogate Protein Structure, Dynamics, and Self- Assembly
University of Pennsylvania ScholarlyCommons Publicly Accessible Penn Dissertations 2016 Using Molecular Constraints and Unnatural Amino Acids to Manipulate and Interrogate Protein Structure, Dynamics, and Self- Assembly Beatrice Markiewicz University of Pennsylvania, [email protected] Follow this and additional works at: https://repository.upenn.edu/edissertations Part of the Biophysics Commons, and the Physical Chemistry Commons Recommended Citation Markiewicz, Beatrice, "Using Molecular Constraints and Unnatural Amino Acids to Manipulate and Interrogate Protein Structure, Dynamics, and Self-Assembly" (2016). Publicly Accessible Penn Dissertations. 1879. https://repository.upenn.edu/edissertations/1879 This paper is posted at ScholarlyCommons. https://repository.upenn.edu/edissertations/1879 For more information, please contact [email protected]. Using Molecular Constraints and Unnatural Amino Acids to Manipulate and Interrogate Protein Structure, Dynamics, and Self-Assembly Abstract Protein molecules can undergo a wide variety of conformational transitions occurring over a series of time and distance scales, ranging from large-scale structural reorganizations required for folding to more localized and subtle motions required for function. Furthermore, the dynamics and mechanisms of such motions and transitions delicately depend on many factors and, as a result, it is not always easy, or even possible, to use existing experimental techniques to arrive at a molecular level understanding of the conformational event of interest. Therefore, this thesis aims to develop and utilize non-natural chemical modification strategies, namely molecular cross-linkers and unnatural amino acids as site-specific spectroscopic probes, in combination with various spectroscopic methods to examine, in great detail, certain aspects of protein folding and functional dynamics, and to manipulate protein self-assemblies. -

Materials Chemistry B Accepted Manuscript
Journal of Materials Chemistry B Accepted Manuscript This is an Accepted Manuscript, which has been through the Royal Society of Chemistry peer review process and has been accepted for publication. Accepted Manuscripts are published online shortly after acceptance, before technical editing, formatting and proof reading. Using this free service, authors can make their results available to the community, in citable form, before we publish the edited article. We will replace this Accepted Manuscript with the edited and formatted Advance Article as soon as it is available. You can find more information about Accepted Manuscripts in the Information for Authors. Please note that technical editing may introduce minor changes to the text and/or graphics, which may alter content. The journal’s standard Terms & Conditions and the Ethical guidelines still apply. In no event shall the Royal Society of Chemistry be held responsible for any errors or omissions in this Accepted Manuscript or any consequences arising from the use of any information it contains. www.rsc.org/materialsB Page 1 of 12 Journal of Materials Chemistry B Journal of Materials Chemistry B RSC Publishing REVIEW Multi-responsive biomaterials and nanobioconjugates from resilin-like protein polymers Cite this: DOI: 10.1039/x0xx00000x Rajkamal Balu, a Jasmin Whittaker, a Naba K. Dutta,*a Christopher M Elvin b and Namita R. Choudhury*a Manuscript Received xxth xxxx xxxx, Accepted xxth xxxxx xxxx Nature, through evolution over millions of years has perfected materials with amazing characteristics and awe-inspiring functionalities that exceed the performance of man-made DOI: 10.1039/x0xx00000x synthetic materials. One such remarkable material is native resilin ─ an extracellular skeletal www.rsc.org/ protein that plays a major role in the jumping, flying, and sound production mechanisms in many insects.