The Impacts of Vehicle Disturbance on NSW Saltmarsh: Implications for Rehabilitation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Historical Riparian Vegetation Changes in Eastern NSW
University of Wollongong Research Online Faculty of Science, Medicine & Health - Honours Theses University of Wollongong Thesis Collections 2016 Historical Riparian Vegetation Changes in Eastern NSW Angus Skorulis Follow this and additional works at: https://ro.uow.edu.au/thsci University of Wollongong Copyright Warning You may print or download ONE copy of this document for the purpose of your own research or study. The University does not authorise you to copy, communicate or otherwise make available electronically to any other person any copyright material contained on this site. You are reminded of the following: This work is copyright. Apart from any use permitted under the Copyright Act 1968, no part of this work may be reproduced by any process, nor may any other exclusive right be exercised, without the permission of the author. Copyright owners are entitled to take legal action against persons who infringe their copyright. A reproduction of material that is protected by copyright may be a copyright infringement. A court may impose penalties and award damages in relation to offences and infringements relating to copyright material. Higher penalties may apply, and higher damages may be awarded, for offences and infringements involving the conversion of material into digital or electronic form. Unless otherwise indicated, the views expressed in this thesis are those of the author and do not necessarily represent the views of the University of Wollongong. Recommended Citation Skorulis, Angus, Historical Riparian Vegetation Changes in Eastern NSW, BSci Hons, School of Earth & Environmental Science, University of Wollongong, 2016. https://ro.uow.edu.au/thsci/120 Research Online is the open access institutional repository for the University of Wollongong. -

Sydneyœsouth Coast Region Irrigation Profile
SydneyœSouth Coast Region Irrigation Profile compiled by Meredith Hope and John O‘Connor, for the W ater Use Efficiency Advisory Unit, Dubbo The Water Use Efficiency Advisory Unit is a NSW Government joint initiative between NSW Agriculture and the Department of Sustainable Natural Resources. © The State of New South Wales NSW Agriculture (2001) This Irrigation Profile is one of a series for New South Wales catchments and regions. It was written and compiled by Meredith Hope, NSW Agriculture, for the Water Use Efficiency Advisory Unit, 37 Carrington Street, Dubbo, NSW, 2830, with assistance from John O'Connor (Resource Management Officer, Sydney-South Coast, NSW Agriculture). ISBN 0 7347 1335 5 (individual) ISBN 0 7347 1372 X (series) (This reprint issued May 2003. First issued on the Internet in October 2001. Issued a second time on cd and on the Internet in November 2003) Disclaimer: This document has been prepared by the author for NSW Agriculture, for and on behalf of the State of New South Wales, in good faith on the basis of available information. While the information contained in the document has been formulated with all due care, the users of the document must obtain their own advice and conduct their own investigations and assessments of any proposals they are considering, in the light of their own individual circumstances. The document is made available on the understanding that the State of New South Wales, the author and the publisher, their respective servants and agents accept no responsibility for any person, acting on, or relying on, or upon any opinion, advice, representation, statement of information whether expressed or implied in the document, and disclaim all liability for any loss, damage, cost or expense incurred or arising by reason of any person using or relying on the information contained in the document or by reason of any error, omission, defect or mis-statement (whether such error, omission or mis-statement is caused by or arises from negligence, lack of care or otherwise). -

1 Murrah Flora Reserves Protect Koalas, but at What
MURRAH FLORA RESERVES PROTECT KOALAS, BUT AT WHAT COST? Published in National Parks Association Winter edition of Nature NSW, 2016 “When we moved to Murrah State Forest over 30 years ago we were besieged by wildlife. The abundance was just amazing. Nothing we planted survived the possums, wallabies, and parrots. The bandicoots dug things up and the wombats caved in the best of fences. We kept bees, and several kinds of sugar gliders came in groups at the first sniff of honey, the bush rats, mice and bats moved in. We could not leave the windows open in summer because of possums at night and goannas by day. The bird life was abundant, and hugely varied. The bush just hummed with life. The river had a stony bottom, lush aquatic plant life, and deep pools with small fish, eels, redfin, bass, and the native cray, the marron. After decades of logging for woodchips, Murrah State Forest became silent; koalas, wildlife, flocks of parrots, potoroo, Christmas beetles and cicadas disappeared, owls and gliders were rarely heard, the rivers silted up with sand, and the diversity of the forest’s tree species was reduced to silver top ash and highly volatile forest casuarinas.”1 Between July 2012 and June 2015 a huge community-based endeavour saw survey teams of contractors, volunteers, Aboriginal Land Council and agency staff, search for koala pellets under more than 30,000 trees at more than 1,000 grid-sites across the 30,000 ha Bermagui to Murrah study area. The results were encouraging, with a population estimated of 30–60 animals2, up from the 23–47 estimated from a similar survey undertaken between 2007 and 20093. -

Effects of Estuarine Acidification on Survival and Growth of the Sydney Rock Oyster Saccostrea Glomerata
EFFECTS OF ESTUARINE ACIDIFICATION ON SURVIVAL AND GROWTH OF THE SYDNEY ROCK OYSTER SACCOSTREA GLOMERATA Michael Colin Dove Submitted in fulfilment of the requirements of the degree of Doctor of Philosophy in The University of New South Wales Geography Program Faculty of the Built Environment The University of New South Wales Sydney, NSW, 2052 April 2003 ACKNOWLEDGEMENTS I would like to thank my supervisor Dr Jes Sammut for his ideas, guidance and encouragement throughout my candidature. I am indebted to Jes for his help with all stages of this thesis, for providing me with opportunities to present this research at conferences and for his friendship. I thank Dr Richard Callinan for his assistance with the histopathology and reviewing chapters of this thesis. I am also very grateful to Laurie Lardner and Ian and Rose Crisp for their invaluable advice, generosity and particular interest in this work. Hastings and Manning River oyster growers were supportive of this research. In particular, I would like to acknowledge the following oyster growers: Laurie and Fay Lardner; Ian and Rose Crisp; Robert Herbert; Nathan Herbert; Stuart Bale; Gary Ruprecht; Peter Clift; Mark Bulley; Chris Bulley; Bruce Fairhall; Neil Ellis; and, Paul Wilson. I am very grateful to Holiday Coast Oysters and Manning River Rock Oysters for providing: the Sydney rock oysters for field and laboratory experiments; storage facilities; equipment; materials; fuel; and, access to resources without reservation. Bruce Fairhall, Paul Wilson, Mark Bulley, Laurie Lardner and Robert Herbert also supplied Sydney rock oysters for this work. I would also like to thank the researchers who gave helpful advice during this study. -
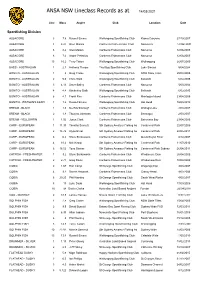
NSW Line Class Records
ANSA NSW Lineclass Records as at 14/03/2021 Line Mass Angler Club Location Date Sportfishing Division ALBACORE 3 7.8 Russell Emms Wollongong Sportfishing Club Kiama Canyons 27/10/2007 ALBACORE 4 8.21 Glen Malam Canberra Fishermans Club Narooma 12/06/2005 ALBACORE 6 8.2 Glen Malam Canberra Fishermans Club Narooma 12/06/2005 ALBACORE 8 7.6 Andre Pretorius Canberra Fishermans Club Narooma 12/06/2005 ALBACORE 15 10.2 Terry Tatton Wollongong Sportfishing Club Wollongong 26/07/2009 BASS - AUSTRALIAN 1 2.1 Anthony Thorpe Trial Bay Sportfishing Club Lake Sinclair 5/04/2004 BONITO - AUSTRALIAN 1 2 Greg Clarke Wollongong Sportfishing Club NSW State Titles 25/04/2005 BONITO - AUSTRALIAN 2 5.6 Chris Stolk Wollongong Sportfishing Club Bellambi 6/02/2005 BONITO - AUSTRALIAN 3 4.13 Steve Bailey Canberra Fishermans Club Narooma 5/03/2004 BONITO - AUSTRALIAN 4 4.4 Kimberley Stolk Wollongong Sportfishing Club Bellambi 6/02/2005 BONITO - AUSTRALIAN 6 4.1 Frank Res Canberra Fishermans Club Montague Island 23/04/2005 BONITO - WATSON'S LEAPI 1 1.2 Russell Emms Wollongong Sportfishing Club Hat Head 18/03/2010 BREAM - BLACK 1 1.6 Mel Marlborough Canberra Fishermans Club Wallaga Lake 2/03/2007 BREAM - BLACK 2 1.8 Thomas Johnston Canberra Fishermans Club Bermagui 2/03/2007 BREAM - YELLOWFIN 1 1.92 Jason Clark Canberra Fishermans Club Batemans Bay 23/04/2005 CARP - EUROPEAN 1 11.35 Timothy Sackett Sth Sydney Amateur Fishing As Centennial Park 22/07/2009 CARP - EUROPEAN 2 14.72 Alyce Ehret Sth Sydney Amateur Fishing As Centennial Park 22/05/2011 CARP - EUROPEAN -

Gov Gaz Week 6 Colour.Indd
3411 Government Gazette OF THE STATE OF NEW SOUTH WALES Number 95 Friday, 8 June 2001 Published under authority by the Government Printing Service LEGISLATION Regulations Exhibited Animals Protection Amendment (Fish Farms and Hatcheries) Regulation 2001 under the Exhibited Animals Protection Act 1986 Her Excellency the Governor, with the advice of the Executive Council, has made the following Regulation under the Exhibited Animals Protection Act 1986. RICHARD AMERY, M.P., Minister forfor Agriculture Agriculture Explanatory note Clause 5 of the Exhibited Animals Protection Regulation 1995 provides for circumstances in which the display of animals is declared not to be an “exhibit” for the purposes of the Exhibited Animals Protection Act 1986. The object of this Regulation is to exclude certain fish that are kept at fish hatcheries and fish farms for commercial food production or re-stocking of lakes, dams or waterways from the definition of “exhibit”. This Regulation is made under the Exhibited Animals Protection Act 1986, including paragraph (c) of the definition of exhibit in section 5 (1) and section 53 (the general regulation-making power). r00-298-p01.819 Page 1 3412 LEGISLATION 8 June 2001 Clause 1 Exhibited Animals Protection Amendment (Fish Farms and Hatcheries) Regulation 2001 Exhibited Animals Protection Amendment (Fish Farms and Hatcheries) Regulation 2001 1 Name of Regulation This Regulation is the Exhibited Animals Protection Amendment (Fish Farms and Hatcheries) Regulation 2001. 2 Amendment of Exhibited Animals Protection Regulation 1995 The Exhibited Animals Protection Regulation 1995 is amended as set out in Schedule 1. 3Notes The explanatory note does not form part of this Regulation. -

Far South Coast Regional Boating Plan
Transport for NSW Regional Boating Plan Far South Coast Region Consultation Draft September 2014 Transport for NSW 18 Lee Street Chippendale NSW 2008 Postal address: PO Box K659 Haymarket NSW 1240 Internet: www.transport.nsw.gov.au Email: [email protected] ISBN Register: 978 -1 -922030 -68 -9 © COPYRIGHT STATE OF NSW THROUGH THE DIRECTOR GENERAL OF TRANSPORT FOR NSW 2014 Extracts from this publication may be reproduced provided the source is fully acknowledged. GHD | Report for Transport for NSW - Regional Boating Plan | i Table of contents 1. Introduction..................................................................................................................................... 4 2. Physical character of the waterways .............................................................................................. 6 2.1 Background .......................................................................................................................... 6 2.2 Clyde catchment .................................................................................................................. 7 2.3 Moruya catchment ............................................................................................................... 8 2.4 Tuross catchment ................................................................................................................ 9 2.5 Bega catchment ................................................................................................................. 11 2.6 Towamba catchment......................................................................................................... -

Bega Valley Shire Local Strategic Planning Statement 2040 PO Box 492, Bega NSW 2550 P
Bega Valley Shire Local Strategic Planning Statement 2040 PO Box 492, Bega NSW 2550 P. (02) 6499 2222 F. (02) 6499 2200 E. [email protected] W. begavalley.nsw.gov.au ABN. 26 987 935 332 DX. 4904 Bega Our Aboriginal Custodians The Bega Valley Shire Council acknowledges the Traditional Custodians of the lands and waters of the Shire, the people of the Yuin nations, and shows respect to elders past, present and emerging. Contents Abbreviations............................................................................................................5 What is the Local Strategic Planning Statement?.......................................................6 Implementation and Monitoring.................................................................................8 Local Values and Priorities.........................................................................................9 Our Place....................................................................................................................10 Shirewide Structure Plan............................................................................................12 Our Community..........................................................................................................14 Planning for the Future..............................................................................................16 Land Use Vision: Bega Valley Shire 2040..................................................................22 Achieving our Vision: ................................................................................................24 -

Fisheries Management (General) Regulation 2019 Under the Fisheries Management Act 1994
New South Wales Fisheries Management (General) Regulation 2019 under the Fisheries Management Act 1994 His Excellency the Lieutenant-Governor, with the advice of the Executive Council, has made the following Regulation under the Fisheries Management Act 1994. ADAM MARSHALL, MP Minister for Agriculture and Western New South Wales Explanatory note The object of this Regulation is to remake, with amendments, the provisions of the Fisheries Management (General) Regulation 2010, which is repealed on 1 September 2019 by section 10 (2) of the Subordinate Legislation Act 1989. This Regulation makes provision with respect to the following: (a) prohibited size fish, bag limits and protected fish and waters, (b) the lawful use of fishing gear, including commercial and recreational nets and traps, (c) rights of priority between commercial and recreational fishers in the use of fishing gear, (d) recreational fishing fees, (e) general matters relating to fisheries management, including offences and restrictions on certain fishing gear and species of fish, (f) share management fisheries, (g) licensing for commercial fishers and fishing boats, (h) the sea urchin and turban shell restricted fishery, the southern fish trawl restricted fishery and the inland restricted fishery, (i) fishing business transfer rules, (j) fish receivers, fish records and fishing business cards, (k) charter fishing management, (l) the protection of aquatic habitats, (m) threatened species conservation, (n) the composition and functions of ministerial advisory councils, (o) enforcement, including the offences under the Act and the regulations for which penalty notices may be issued and the amounts of the penalties payable, (p) other minor and savings provisions. Published LW 23 August 2019 (2019 No 407) Fisheries Management (General) Regulation 2019 [NSW] Explanatory note This Regulation is made under the Fisheries Management Act 1994, including the sections referred to in the Regulation and section 289 (the general regulation-making power). -

Oyster Industry Projects
Vol. 3. November 2011 [Email to subscribe directly] [Email to unsubscribe] Welcome to the Oyster Newsletter At the request of many oyster industry representatives, this newsletter provides an update on national and international news, events and research that may be of interest to the oyster industry. It is provided to you free, and has the simple goal of providing relevant information to industry. It does not aim to replace those newsletters currently published for the oyster industry, but to supply and collate additional information that isn’t readily available into one space. We encourage farmers to circulate it widely through the industry - print it off and pass it around your workers. If you would like to unsubscribe, please click on the link above, or email [email protected]. Since this newsletter is for your information and as a tool to improve communication across oyster regions, we would appreciate any contributions, ideas for future editions, stories or projects in which you are involved, photographs, or any other feedback... Happy oystering! Andy (OceanWatch Australia) & Ana (University of Wollongong) Oyster Industry Projects NSW Oyster EMS Projects The future of the oyster industry depends not only on a healthy environment, but also on the ability to demonstrate that growers are utilising the environment in a responsible and sustainable manner. One way of doing this is to develop an Environmental Management System (EMS) to show how environmental risks, impacts and opportunities are managed. The process of EMS development allows oyster farmers to identify risks arising from internal, on- farm activities and also from external, catchment-based activities. -

Far South Coast Regional Boating Plan Has Been Developed As Part of a Major NSW Government Initiative to Boost the Experience of Recreational Boating Across the State
Transport for NSW Regional Boating Plan Far South Coast Region February 2015 Transport for NSW 18 Lee Street Chippendale NSW 2008 Postal address: PO Box K659 Haymarket NSW 1240 Internet: www.transport.nsw.gov.au Email: [email protected] ISBN Register: 978 -1 -922030 -68 -9 © COPYRIGHT STATE OF NSW THROUGH THE DIRECTOR GENERAL OF TRANSPORT FOR NSW 2014 Extracts from this publication may be reproduced provided the source is fully acknowledged. Report for Transport for NSW - Regional Boating Plan | i Table of contents 1. Introduction..................................................................................................................................... 4 2. Physical character of the waterways .............................................................................................. 6 2.1 Background .......................................................................................................................... 6 2.2 Clyde catchment/region ....................................................................................................... 7 2.3 Moruya catchment ............................................................................................................... 8 2.4 Tuross catchment ................................................................................................................ 9 2.5 Bega catchment ................................................................................................................. 11 2.6 Towamba catchment......................................................................................................... -

Shallow Water Fish Communities of New South Wales South Coast Estuaries
FRDC Project 97/204: Shallow Water Fish Communities of New South Wales South Coast Estuaries R.J. West and M.V. Jones University of Wollongong Ocean and Coastal Research Centre Report Series Nos. 2001/1 January 2001 ISBN: 0 86418 739 4 FRDC Project 97/204 HISTORICAL NOTE Extracts from H.C. Dannevig (1906): “Correlation between our Home Fisheries and the Salt Water Lakes on the South Coast” “When visiting the Lakes district on the South Coast some little time ago, in company with the Hon. J.H. Want, a member of the Board, I had an opportunity of inspecting the principal nurseries from which our fish mainly migrate, and we also got a fair insight into the manner in which these waters are being worked. There are over forty salt-water lakes from St. Georges Basin to the border, with an area each of 30 acres or more, the total expanse being about 32,000 acres. Some of these waters are constantly open to the sea, others occasionally closed, while a few are only connected to the sea for a few weeks at a time, and then only at long intervals. It follows that the first-mentioned only are available to their full extent as natural nurseries, and the others with a closing entrance are more or less handicapped." Shallow water fishing method. Citation: West, R.J. and Jones, M.V. 2001. FRDC 97/204 Final Report: Shallow water fish communities of New South Wales south coast estuaries. Report Series Nos. 2001/1. Ocean and Coastal Research Centre, University of Wollongong, Australia.