Long QT Syndrome in Neonates
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Syndrome of Alternating Bradycardia and Tachycardia by D
Br Heart J: first published as 10.1136/hrt.16.2.208 on 1 April 1954. Downloaded from THE SYNDROME OF ALTERNATING BRADYCARDIA AND TACHYCARDIA BY D. S. SHORT From the National Heart Hospita. Received September 15, 1953 Among the large number of patients suffering from syncopal attacks who attended the National Heart Hospital during a four-year period, there were four in whom examination revealed sinus bradycardia alternating with prolonged phases of auricular tachycardia. These patients presented a difficult problem in treatment. Each required at least one admission to hospital and in one case the symptoms were so intractable as to necessitate six admissions in five years. Two patients had mitral valve disease, one of them with left bundle branch block. One had aortic valve sclerosis while the fourth had no evidence of heart disease. THE HEART RATE The sinus rate usually lay between 30 and 50 a minute, a rate as slow as 22 a minute being observed in one patient (Table I). Sinus arrhythmia was noted in all four patients, wandering of TABLE I http://heart.bmj.com/ RATE IN SINus RHYTHM AND IN AURICULAR TACHYCARDIA Rate in Case Age Sex Associated Rate in auricular tachycardia heart disease sinus rhythm Auricular Venliicular 1 65 M Aortic valve sclerosis 28-48 220-250 60-120 2 47 F Mitral valve disease 35-75 180-130 90-180 on September 26, 2021 by guest. Protected copyright. 3 38 F Mitral valve disease 22-43 260 50-65 4 41 F None 35-45 270 110 the pacemaker in three, and periods of sinus standstill in two (Fig. -

Basic Rhythm Recognition
Electrocardiographic Interpretation Basic Rhythm Recognition William Brady, MD Department of Emergency Medicine Cardiac Rhythms Anatomy of a Rhythm Strip A Review of the Electrical System Intrinsic Pacemakers Cells These cells have property known as “Automaticity”— means they can spontaneously depolarize. Sinus Node Primary pacemaker Fires at a rate of 60-100 bpm AV Junction Fires at a rate of 40-60 bpm Ventricular (Purkinje Fibers) Less than 40 bpm What’s Normal P Wave Atrial Depolarization PR Interval (Normal 0.12-0.20) Beginning of the P to onset of QRS QRS Ventricular Depolarization QRS Interval (Normal <0.10) Period (or length of time) it takes for the ventricles to depolarize The Key to Success… …A systematic approach! Rate Rhythm P Waves PR Interval P and QRS Correlation QRS Rate Pacemaker A rather ill patient……… Very apparent inferolateral STEMI……with less apparent complete heart block RATE . Fast vs Slow . QRS Width Narrow QRS Wide QRS Narrow QRS Wide QRS Tachycardia Tachycardia Bradycardia Bradycardia Regular Irregular Regular Irregular Sinus Brady Idioventricular A-Fib / Flutter Bradycardia w/ BBB Sinus Tach A-Fib VT PVT Junctional 2 AVB / II PSVT A-Flutter SVT aberrant A-Fib 1 AVB 3 AVB A-Flutter MAT 2 AVB / I or II PAT PAT 3 AVB ST PAC / PVC Stability Hypotension / hypoperfusion Altered mental status Chest pain – Coronary ischemic Dyspnea – Pulmonary edema Sinus Rhythm Sinus Rhythm P Wave PR Interval QRS Rate Rhythm Pacemaker Comment . Before . Constant, . Rate 60-100 . Regular . SA Node Upright in each QRS regular . Interval =/< leads I, II, . Look . Interval .12- .10 & III alike .20 Conduction Image reference: Cardionetics/ http://www.cardionetics.com/docs/healthcr/ecg/arrhy/0100_bd.htm Sinus Pause A delay of activation within the atria for a period between 1.7 and 3 seconds A palpitation is likely to be felt by the patient as the sinus beat following the pause may be a heavy beat. -

Basic Cardiac Rhythms – Identification and Response Module 1 ANATOMY, PHYSIOLOGY, & ELECTRICAL CONDUCTION Objectives
Basic Cardiac Rhythms – Identification and Response Module 1 ANATOMY, PHYSIOLOGY, & ELECTRICAL CONDUCTION Objectives ▪ Describe the normal cardiac anatomy and physiology and normal electrical conduction through the heart. ▪ Identify and relate waveforms to the cardiac cycle. Cardiac Anatomy ▪ 2 upper chambers ▪ Right and left atria ▪ 2 lower chambers ▪ Right and left ventricle ▪ 2 Atrioventricular valves (Mitral & Tricuspid) ▪ Open with ventricular diastole ▪ Close with ventricular systole ▪ 2 Semilunar Valves (Aortic & Pulmonic) ▪ Open with ventricular systole ▪ Open with ventricular diastole The Cardiovascular System ▪ Pulmonary Circulation ▪ Unoxygenated – right side of the heart ▪ Systemic Circulation ▪ Oxygenated – left side of the heart Anatomy Coronary Arteries How The Heart Works Anatomy Coronary Arteries ▪ 2 major vessels of the coronary circulation ▪ Left main coronary artery ▪ Left anterior descending and circumflex branches ▪ Right main coronary artery ▪ The left and right coronary arteries originate at the base of the aorta from openings called the coronary ostia behind the aortic valve leaflets. Physiology Blood Flow Unoxygenated blood flows from inferior and superior vena cava Right Atrium Tricuspid Valve Right Ventricle Pulmonic Valve Lungs Through Pulmonary system Physiology Blood Flow Oxygenated blood flows from the pulmonary veins Left Atrium Mitral Valve Left Ventricle Aortic Valve Systemic Circulation ▪ Blood Flow Through The Heart ▪ Cardiology Rap Physiology ▪ Cardiac cycle ▪ Represents the actual time sequence between -

Case Report Chagas Cardiomyopathy Presenting As Symptomatic Bradycardia: an Underappreciated Emerging Public Health Problem in the United States
Hindawi Case Reports in Cardiology Volume 2017, Article ID 5728742, 5 pages https://doi.org/10.1155/2017/5728742 Case Report Chagas Cardiomyopathy Presenting as Symptomatic Bradycardia: An Underappreciated Emerging Public Health Problem in the United States Richard Jesse Durrance,1 Tofura Ullah,1 Zulekha Atif,1 William Frumkin,2 and Kaushik Doshi1 1 Department of Internal Medicine, Jamaica Hospital Medical Center, 8900 Van Wyck Expressway, Jamaica, NY 11418, USA 2Department of Cardiology, Jamaica Hospital Medical Center, 8900 Van Wyck Expressway, Jamaica, NY 11418, USA Correspondence should be addressed to Richard Jesse Durrance; [email protected] Received 13 February 2017; Accepted 18 July 2017; Published 16 August 2017 Academic Editor: Aiden Abidov Copyright © 2017 Richard Jesse Durrance et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Chagas cardiomyopathy (CCM) is traditionally considered a disease restricted to areas of endemicity. However, an estimated 300,000 people living in the United States today have CCM, of which its majority is undiagnosed. Wepresent a case of CCM acquired in an endemic area and detected in its early stage. A 42-year-old El Salvadoran woman presented with recurrent chest pain and syncopal episodes. Significant family history includes a sister inEl Salvador who also began suffering similar episodes. Physical exam and ancillary studies were only remarkable for sinus bradycardia. The patient was diagnosed with symptomatic sinus bradycardia and a pacemaker was placed. During her hospital course, Chagas serology was ordered given the epidemiological context from which she came. -

Sinus Bradycardia
British Heart Journal, I97I, 33, 742-749. Br Heart J: first published as 10.1136/hrt.33.5.742 on 1 September 1971. Downloaded from Sinus bradycardia Dennis Eraut and David B. Shaw From the Cardiac Department, Royal Devon and Exeter Hospital, Exeter, Devon This paper presents thefeatures of 46 patients with unexplained bradycardia. Patients were ad- mitted to the study if their resting atrial rate was below 56 a minute on two consecutive occasions. Previous electrocardiograms and the response to exercise, atropine, and isoprenaline were studied. The ages of thepatients variedfrom I3 to 88years. Only 8 had a past history ofcardiovascular disease other than bradycardia, but 36 hJd syncopal or dizzy attacks. Of the 46 patients, 35 had another arrhythmia in addition to bradycardia; at some stage, i6 had sinus arrest, i.5 hadjunc- tional rhythm, 12 had fast atrial arrhythmia, I6 had frequent extrasystoles, and 6 had atrio- ventricular block. None had the classical features of sinoatrial block. Arrhythmias were often produced by exercise, atropine, or isoprenaline. Drug treatment was rarely satisfactory, but only i patient needed a permanent pacemaker. It is suggested that the majority of the patients were suffering from a pathological form of sinus bradycardia. The aetiology remains unproven, but the most likely explanation is a loss of the inherent rhythmicity of the sinoatrial node due to a primary degenerative disease. The descriptive title of 'the lazy sinus syndrome' is suggested. copyright. Bradycardia with a slow atrial rate is usually attempt to define the clinical syndrome of regarded as an innocent condition common in bradycardia with a pathologically slow atrial certain types of well-trained athlete, but occa- rate and to clarify the nature of the arrhyth- sionally it may occur in patients with symp- mia. -
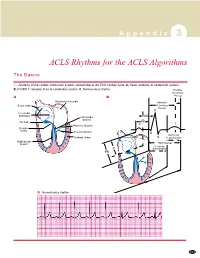
ACLS Rhythms for the ACLS Algorithms
A p p e n d i x 3 ACLS Rhythms for the ACLS Algorithms The Basics 1. Anatomy of the cardiac conduction system: relationship to the ECG cardiac cycle. A, Heart: anatomy of conduction system. B, P-QRS-T complex: lines to conduction system. C, Normal sinus rhythm. Relative Refractory A B Period Bachmann’s bundle Absolute Sinus node Refractory Period R Internodal pathways Left bundle AVN branch AV node PR T Posterior division P Bundle of His Anterior division Q Ventricular Purkinje fibers S Repolarization Right bundle branch QT Interval Ventricular P Depolarization PR C Normal sinus rhythm 253 A p p e n d i x 3 The Cardiac Arrest Rhythms 2. Ventricular Fibrillation/Pulseless Ventricular Tachycardia Pathophysiology ■ Ventricles consist of areas of normal myocardium alternating with areas of ischemic, injured, or infarcted myocardium, leading to chaotic pattern of ventricular depolarization Defining Criteria per ECG ■ Rate/QRS complex: unable to determine; no recognizable P, QRS, or T waves ■ Rhythm: indeterminate; pattern of sharp up (peak) and down (trough) deflections ■ Amplitude: measured from peak-to-trough; often used subjectively to describe VF as fine (peak-to- trough 2 to <5 mm), medium-moderate (5 to <10 mm), coarse (10 to <15 mm), very coarse (>15 mm) Clinical Manifestations ■ Pulse disappears with onset of VF ■ Collapse, unconsciousness ■ Agonal breaths ➔ apnea in <5 min ■ Onset of reversible death Common Etiologies ■ Acute coronary syndromes leading to ischemic areas of myocardium ■ Stable-to-unstable VT, untreated ■ PVCs with -

The Bradycardia-Tachycardia Syndrome Treatment with Cardiac Drugs and Adrenal Corticosteroid Junichi FUJII, M.D., Nobumitsu TAKA
The Bradycardia-Tachycardia Syndrome Treatment with Cardiac Drugs and Adrenal Corticosteroid Junichi FUJII, M.D., Nobumitsu TAKAHASHI,M.D., and Kazuzo KATO, M.D. Seven patients of S-A block complicated by tachycardic paroxysms of atrial fibrillation or flutter were described and the medical treatment in this syndrome was reappraised. Damage to S-A node and adjacent atrial tissue was assumed in all patients. All the patients had syncopal attacks associated with cardiac arrest occurring especially at the termina- tion of tachycardia. Overdrive suppression of diseased S-A node and lower automatic pacemakers was demonstrated by ECG recordings. The term "bradycardia-tachycardia syndrome" or "syndrome of alternating bradycardia and tachycardia" seemed appropriate. In spite of difficulty of medical treatment reiterated by previous de- scriptions, 6 of 7 patients were improved with drug therapy, including adrenal corticosteroid. Adrenal corticosteroid in combination with or- ciprenaline or belladonna alkaloids was most helpful among the drugs used. Obviously, pacemaker implantation should be performed without delay in patients with frequent and prolonged attacks of syncope. But not all patients have need of pacemaker implantation. A trial of drug therapy may be permitted in many patients of this syndrome before in- troduction of pacemaker. Additional Indexing Words: Sick sinus syndrome S-A block Atrial tachyarrhythmias Syn- cope Overdrive suppression ECENTLY there have been some reports concerning the patients with S-A block or sinus bradycardia accompanied by paroxysmal atrial tachy- arrhythmias such as fibrillation, flutter and paroxysmal tachycardia. As pointed out by several authors, these patients repeatedly exhibited syncopal attacks associated with asystole following termination of the tachycardia. -

Wolff–Parkinson–White Syndrome Unmasked by Atrial Pacing in A
EP CASE REPORT ............................................................................................................................................................................. Wolff–Parkinson–White syndrome unmasked by atrial pacing in a patient with cardiac sarcoidosis Francisca Caetano1*, Se´rgio Barra1, and Diogo Cavaco2 5 1Department of Cardiology, Centro Hospitalar e Universita´rio de Coimbra, 3030-775 Coimbra, Portugal; and 2Department of Cardiology, Centro Hospitalar Lisboa Ocidental - Hospital Santa Cruz, Lisboa * Corresponding author. Tel:+351 239 800 100; fax: +351 239 814 779. E-mail address: [email protected] Male patient, 55 years old, diagnosed with cardiac sarcoidosis, underwent pacemaker implantation for sick sinus syndrome. After implant- 10 ation, electrocardiogram showed atrial pacing, short PR interval, and slurred upstroke of the spontaneous QRS complex. During pace- maker follow-up, several episodes of sustained tachyarrhythmia were documented. Electrophysiological study was performed disclosing an overt left postero-septal accessory pathway and successful ablation was performed. Case report 15 A 55-year old male patient diagnosed with pulmonary and ocular sarcoidosis at the age of 50 and chronically treated with corticosteroids was evaluated for the first time at the Cardiology Department 5 years following initial diagnosis for symptomatic sinus bradycardia (com- plaints of dyspnoea NYHA II/IV, dizziness, and pre-syncope). Ancillary tests, namely cardiac magnetic resonance, confirmed cardiac involvement. -

Management for a Girl with LQTS and Sinus Bradycardia
2015 춘계통합학술대회 Management for a Girl With LQTS and Sinus Bradycardia 아주 의대 황 교 승 Management for a Girl With Long QT syndrome (LQTS) and Sinus Bradycardia Management for a Girl With LQTS and Sinus Bradycardia Action Potential and Electrocardiogram Normal Values for Durations of ECG Waves and Intervals in Adults Braunwald’s Heart Disease, 8th The corrected QT interval (QTc)= QT interval/√RR (Bazett’s formula) Congenital LQTS Historical features • Syncope • Congenital deafness • Family history of long QT syndrome • Unexplained sudden death in family member b age 30 ECG features • Prolonged QTc interval • Torsades de pointes • T-wave alternans • Notched T-waves in 3 or more leads • Bradycardia * The best example of genotype-phenotype correlation Notched T Waves T Wave Alternans Eur Heart J. 2013 Molecular Basis of LQTS J Am Coll Cardiol. 2013 July 16; 62(3): 169–180 P J Schwartz Circulation. 1993;88:782-784 European Heart Journal (2013) 34, 3109–3116 LQTS Diagnosis Europace (2013) 15, 1389–1406 Distribution of QTc Values Among Individuals With and without LQTS Circulation. 2007;115:2613-2620 Epinephrine QT Stress Test * 25% to 50% of patients with LQT1, LQT2, or LQT3: nondiagnostic resting QTc Circulation. 2006;113:1385-1392 * One-third of patients with LQTS: normal QT interval on at least one ECG Circulation 2007;115:2613 1. A 25-minute infusion protocol (0.025 to 0.3 g · kg1 · min1). Circulation. 2006;113:1385-1392 2. A bolus injection of epinephrine (0.1 g/kg)--- continuous infusion (0.1 g/kg/min) Heart Rhythm (2004) 3, 276–283 Diagnostic Outcome of LQTS Referral Cohort Circulation. -
Heart Rhythms
Heart rhythms Coronary heart disease is the UK’s single biggest killer. For over 50 years we’ve pioneered research that’s transformed the lives of people living with heart and circulatory conditions. Our work has been central to the discoveries of vital treatments that are changing the fight against heart disease. But so many people still need our help. From babies born with life-threatening heart problems to the many Mums, Dads and Grandparents who survive a heart attack and endure the daily battles of heart failure. Join our fight for every heartbeat in the UK. Every pound raised, minute of your time and donation to our shops will help make a difference to people’s lives. ©British Heart Foundation 2013, registered charity in England and Wales (225971) and in Scotland (SCO39426) HIS14/0512 About the British Heart Foundation The British Heart Foundation (BHF) is the nation’s heart charity, saving lives through pioneering research, patient care and vital information. What you can do for us We rely on donations of time and money to continue our life-saving work. If you would like to make a donation, please: • call our donation hotline on 0300 330 3322 • visit bhf.org.uk/donate, or • post it to us at the address on the back cover. If you wish to make a gift to the BHF in your will, call 0844 847 2787 or email [email protected] and ask for our free booklet, My generation. For other ways to support our work, see bhf.org.uk/ supportus You may find other useful information on our website at: bhf.org.uk Contents About this booklet ....................................................................... -
What Are ECG Normal Variants?
Many common ECG findings What are ECG are normal variants and are not cause for deferment, unless the pilot is Normal Variants? symptomatic or there are page 1 of 6 other concerns. Let’s look at a few examples of ECG Normal Variants... Sinus Bradycardia Sinus bradycardia is a normal variant if the pilot is 49 or younger, and their heart rate is greater than 44 beats per minute. At age 50 and older, their heart rate must be greater than 48 beats per minute. Sinus Tachycardia Sinus tachycardia is a normal variant if the pilot’s heart rate is less than 110 beats per minute. Sinus Arrhythmia Sinus arrhythmia is a normal variant. Many common ECG findings What are ECG are normal variants and are not cause for deferment, unless the pilot is Normal Variants? symptomatic or there are page 2 of 6 other concerns. Let’s look at a few examples of ECG Normal Variants... Low Atrial Rhythm Low atrial rhythm is a normal variant when there are upright P waves in the AVR lead, and inverted P’s in other limb leads with a short PR interval. Ectopic Atrial Rhythm With ectopic atrial rhythm, the S.A. node rhythm originates in multiple areas of the atria. Wandering Atrial Pacemaker A wandering atrial pacemaker looks similar to ectopic atrial rhythm, depending on the interpretive output of your diagnostic equipment. Many common ECG findings What are ECG are normal variants and are not cause for deferment, unless the pilot is Normal Variants? symptomatic or there are page 3 of 6 other concerns. -

Tachycardia-Bradycardia Syndrome in a Patient with Atrial Fibrillation
Case Report pISSN 2005-6419·eISSN 2005-7563 KJA Tachycardia-bradycardia Korean Journal of Anesthesiology syndrome in a patient with atrial fibrillation -a case report- Sung Hwan Choi, Sung Lark Choi, Bong Yeong Lee, and Mi Ae Jeong Department of Anesthesiology and Pain Medicine, Hanyang University Hospital, Seoul, Korea An 83-year-old woman was scheduled for a second transurethral resection of a bladder tumor. The preoperative elec- trocardiogram evaluation revealed atrial fibrillation with a slow ventricular response (ventricular rate: 59 /min). After intravenous injection of 1% lidocaine 40 mg and propofol 60 mg, the ventricular rate increased to 113 beats/min and then fell rapidly to 27 beats/min. Blood pressure was 70/40 mmHg. Later an atrial fibrillation rhythm, with a ventricular rate of 100–130 beats/min, was observed together with a sinus pause and sinus rhythm with a ventricular rate of 40–50 beats/min. An external pacemaker was applied and set at 60 mA, 40 counts. After the patient regained consciousness, she presented an alert mental state and had no chest symptoms. She was discharged 2 weeks later without complications after insertion of a permanent pacemaker. Key Words: Artificial cardaic pacing, Atrial fibrillation, Sick sinus syndrome. Tachycardia-bradycardia syndrome is a variant of sick sinus with this arrhythmia require treatment and care because of the syndrome (caused by a functional defect of the sinus node, the risk of ischemic heart disease, hypertensive heart disease, cardi- primary pacemaker of the heart) in which slow and fast ar- ac failure and embolism [4]. Also, patients with slow ventricular rhythmias alternate [1].