A Multi-Angled Approach to Discover and Improve Skeletal Muscle Stem Cell Therapies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Human and Mouse CD Marker Handbook Human and Mouse CD Marker Key Markers - Human Key Markers - Mouse
Welcome to More Choice CD Marker Handbook For more information, please visit: Human bdbiosciences.com/eu/go/humancdmarkers Mouse bdbiosciences.com/eu/go/mousecdmarkers Human and Mouse CD Marker Handbook Human and Mouse CD Marker Key Markers - Human Key Markers - Mouse CD3 CD3 CD (cluster of differentiation) molecules are cell surface markers T Cell CD4 CD4 useful for the identification and characterization of leukocytes. The CD CD8 CD8 nomenclature was developed and is maintained through the HLDA (Human Leukocyte Differentiation Antigens) workshop started in 1982. CD45R/B220 CD19 CD19 The goal is to provide standardization of monoclonal antibodies to B Cell CD20 CD22 (B cell activation marker) human antigens across laboratories. To characterize or “workshop” the antibodies, multiple laboratories carry out blind analyses of antibodies. These results independently validate antibody specificity. CD11c CD11c Dendritic Cell CD123 CD123 While the CD nomenclature has been developed for use with human antigens, it is applied to corresponding mouse antigens as well as antigens from other species. However, the mouse and other species NK Cell CD56 CD335 (NKp46) antibodies are not tested by HLDA. Human CD markers were reviewed by the HLDA. New CD markers Stem Cell/ CD34 CD34 were established at the HLDA9 meeting held in Barcelona in 2010. For Precursor hematopoetic stem cell only hematopoetic stem cell only additional information and CD markers please visit www.hcdm.org. Macrophage/ CD14 CD11b/ Mac-1 Monocyte CD33 Ly-71 (F4/80) CD66b Granulocyte CD66b Gr-1/Ly6G Ly6C CD41 CD41 CD61 (Integrin b3) CD61 Platelet CD9 CD62 CD62P (activated platelets) CD235a CD235a Erythrocyte Ter-119 CD146 MECA-32 CD106 CD146 Endothelial Cell CD31 CD62E (activated endothelial cells) Epithelial Cell CD236 CD326 (EPCAM1) For Research Use Only. -

Viewed Under 23 (B) Or 203 (C) fi M M Male Cko Mice, and Largely Unaffected Magni Cation; Scale Bars, 500 M (B) and 50 M (C)
BRIEF COMMUNICATION www.jasn.org Renal Fanconi Syndrome and Hypophosphatemic Rickets in the Absence of Xenotropic and Polytropic Retroviral Receptor in the Nephron Camille Ansermet,* Matthias B. Moor,* Gabriel Centeno,* Muriel Auberson,* † † ‡ Dorothy Zhang Hu, Roland Baron, Svetlana Nikolaeva,* Barbara Haenzi,* | Natalya Katanaeva,* Ivan Gautschi,* Vladimir Katanaev,*§ Samuel Rotman, Robert Koesters,¶ †† Laurent Schild,* Sylvain Pradervand,** Olivier Bonny,* and Dmitri Firsov* BRIEF COMMUNICATION *Department of Pharmacology and Toxicology and **Genomic Technologies Facility, University of Lausanne, Lausanne, Switzerland; †Department of Oral Medicine, Infection, and Immunity, Harvard School of Dental Medicine, Boston, Massachusetts; ‡Institute of Evolutionary Physiology and Biochemistry, St. Petersburg, Russia; §School of Biomedicine, Far Eastern Federal University, Vladivostok, Russia; |Services of Pathology and ††Nephrology, Department of Medicine, University Hospital of Lausanne, Lausanne, Switzerland; and ¶Université Pierre et Marie Curie, Paris, France ABSTRACT Tight control of extracellular and intracellular inorganic phosphate (Pi) levels is crit- leaves.4 Most recently, Legati et al. have ical to most biochemical and physiologic processes. Urinary Pi is freely filtered at the shown an association between genetic kidney glomerulus and is reabsorbed in the renal tubule by the action of the apical polymorphisms in Xpr1 and primary fa- sodium-dependent phosphate transporters, NaPi-IIa/NaPi-IIc/Pit2. However, the milial brain calcification disorder.5 How- molecular identity of the protein(s) participating in the basolateral Pi efflux remains ever, the role of XPR1 in the maintenance unknown. Evidence has suggested that xenotropic and polytropic retroviral recep- of Pi homeostasis remains unknown. Here, tor 1 (XPR1) might be involved in this process. Here, we show that conditional in- we addressed this issue in mice deficient for activation of Xpr1 in the renal tubule in mice resulted in impaired renal Pi Xpr1 in the nephron. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Supplementary Table 3
Supplemental Table 1 M e13 ∆∆Ct e13 M e15 ∆∆Ct e15 chromogranin A -3,26 (9,6 ↓ ) -6,29 (78 ↓ ) -2,56 (5,9 ↓ ) -6,57 (95 ↓ ) crystallin, beta A2 -0,95 (1,9 ↓ ) -4,57 (24 ↓ ) -1,82 (3,5 ↓ ) -4 (16 ↓ ) cyclin-dependent kinase inhibitor 1A (P21) -1,15 (2,2 ↓ ) -1,41 (2,7 ↓ ) -0,36 (1,3 ↓ ) 0,29 (1,2 ↑ ) cytochrome P450, family 4, subfamily b, polypeptide 1 -0,68 (1,6 ↓ ) 0,16 (1,1 ↑ ) -0,56 (1,5 ↓ ) -0,08 (1,1 ↓ ) myelin transcription factor 1 -1,28 (2,4 ↓ ) -2,62 (6,1 ↓ ) -1,46 (2,8 ↓ ) -3,59 (12 ↓ ) neurogenic differentiation 2 -0,06 (1,0 → ) NA -1,34 (2,5 ↓ ) NA neuronatin 0,14 (1,1 ↑ ) 0,12 (1,1 ↑ ) -0,79 (1,7 ↓ ) -2,02 (4,1 ↓ ) protocadherin 21 -1,62 (3,1 ↓ ) -5,71 (52 ↓ ) -1,77 (3,4 ↓ ) -6,41 (85 ↓ ) regulated endocrine-specific protein 18 -2,1 (4,3 ↓ ) -4,73 (27 ↓ ) -1,55 (2,9 ↓ ) -5,09 (34 ↓ ) retinol binding protein 4, plasma -1,68 (3,2 ↓ ) -1,52 (2,9 ↓ ) -1,53 (2,9 ↓ ) -2,15 (4,4 ↓ ) rhomboid, veinlet-like 4 (Drosophila) -1,14 (2,2 ↓ ) -0,29 (1,2 ↓ ) -1,09 (2,1 ↓ ) -0,58 (1,5 ↓ ) sestrin 2 -0,78 (1,7 ↓ ) -0,84 (1,8 ↓ ) -0,67 (1,6 ↓ ) -0,61 (1,5 ↓ ) synaptotagmin 13 -1,63 (3,1 ↓ ) -2,59 (6,0 ↓ ) -1,77 (3,4 ↓ ) -2,71 (6,5 ↓ ) t-complex protein 11 -0,48 (1,4 ↓ ) -1,35 (2,5 ↓ ) -0,68 (1,6 ↓ ) -2,83 (7,1 ↓ ) -0,62 (1,5 ↓ ) -0,76 (1,7 ↓ ) transmembrane 4 superfamily member 2 -0,29 (1,2 ↓ ) -0,55 (1,5 ↓ ) -0,67 (1,6 ↓ ) -0,38 (1,3 ↓ ) 2510004L01Rik -0,7 (1,6 ↓ ) -1,58 (3,0 ↓ ) -0,07 (1,0 → ) 0,16 (1,1 ↑ ) C81234 -3,12 (8,7 ↓ ) -7,75 (215 ↓ ) -2,29 (4,9 ↓ ) -4,86 (29 ↓ ) Insulin 2 NM -9,89 (948 ↓ ) NM -14,2 (18820 ↓ ) Neurogenin 3 NM NA -

CD Markers Are Routinely Used for the Immunophenotyping of Cells
ptglab.com 1 CD MARKER ANTIBODIES www.ptglab.com Introduction The cluster of differentiation (abbreviated as CD) is a protocol used for the identification and investigation of cell surface molecules. So-called CD markers are routinely used for the immunophenotyping of cells. Despite this use, they are not limited to roles in the immune system and perform a variety of roles in cell differentiation, adhesion, migration, blood clotting, gamete fertilization, amino acid transport and apoptosis, among many others. As such, Proteintech’s mini catalog featuring its antibodies targeting CD markers is applicable to a wide range of research disciplines. PRODUCT FOCUS PECAM1 Platelet endothelial cell adhesion of blood vessels – making up a large portion molecule-1 (PECAM1), also known as cluster of its intracellular junctions. PECAM-1 is also CD Number of differentiation 31 (CD31), is a member of present on the surface of hematopoietic the immunoglobulin gene superfamily of cell cells and immune cells including platelets, CD31 adhesion molecules. It is highly expressed monocytes, neutrophils, natural killer cells, on the surface of the endothelium – the thin megakaryocytes and some types of T-cell. Catalog Number layer of endothelial cells lining the interior 11256-1-AP Type Rabbit Polyclonal Applications ELISA, FC, IF, IHC, IP, WB 16 Publications Immunohistochemical of paraffin-embedded Figure 1: Immunofluorescence staining human hepatocirrhosis using PECAM1, CD31 of PECAM1 (11256-1-AP), Alexa 488 goat antibody (11265-1-AP) at a dilution of 1:50 anti-rabbit (green), and smooth muscle KD/KO Validated (40x objective). alpha-actin (red), courtesy of Nicola Smart. PECAM1: Customer Testimonial Nicola Smart, a cardiovascular researcher “As you can see [the immunostaining] is and a group leader at the University of extremely clean and specific [and] displays Oxford, has said of the PECAM1 antibody strong intercellular junction expression, (11265-1-AP) that it “worked beautifully as expected for a cell adhesion molecule.” on every occasion I’ve tried it.” Proteintech thanks Dr. -

Supplementary Table S4. FGA Co-Expressed Gene List in LUAD
Supplementary Table S4. FGA co-expressed gene list in LUAD tumors Symbol R Locus Description FGG 0.919 4q28 fibrinogen gamma chain FGL1 0.635 8p22 fibrinogen-like 1 SLC7A2 0.536 8p22 solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 DUSP4 0.521 8p12-p11 dual specificity phosphatase 4 HAL 0.51 12q22-q24.1histidine ammonia-lyase PDE4D 0.499 5q12 phosphodiesterase 4D, cAMP-specific FURIN 0.497 15q26.1 furin (paired basic amino acid cleaving enzyme) CPS1 0.49 2q35 carbamoyl-phosphate synthase 1, mitochondrial TESC 0.478 12q24.22 tescalcin INHA 0.465 2q35 inhibin, alpha S100P 0.461 4p16 S100 calcium binding protein P VPS37A 0.447 8p22 vacuolar protein sorting 37 homolog A (S. cerevisiae) SLC16A14 0.447 2q36.3 solute carrier family 16, member 14 PPARGC1A 0.443 4p15.1 peroxisome proliferator-activated receptor gamma, coactivator 1 alpha SIK1 0.435 21q22.3 salt-inducible kinase 1 IRS2 0.434 13q34 insulin receptor substrate 2 RND1 0.433 12q12 Rho family GTPase 1 HGD 0.433 3q13.33 homogentisate 1,2-dioxygenase PTP4A1 0.432 6q12 protein tyrosine phosphatase type IVA, member 1 C8orf4 0.428 8p11.2 chromosome 8 open reading frame 4 DDC 0.427 7p12.2 dopa decarboxylase (aromatic L-amino acid decarboxylase) TACC2 0.427 10q26 transforming, acidic coiled-coil containing protein 2 MUC13 0.422 3q21.2 mucin 13, cell surface associated C5 0.412 9q33-q34 complement component 5 NR4A2 0.412 2q22-q23 nuclear receptor subfamily 4, group A, member 2 EYS 0.411 6q12 eyes shut homolog (Drosophila) GPX2 0.406 14q24.1 glutathione peroxidase -

Identification of Tetraspanin-7 As a Target of Autoantibodies in Type 1 Diabetes
Page 1 of 35 Diabetes Identification of Tetraspanin-7 as a Target of Autoantibodies in Type 1 Diabetes Running title: Tetraspanin-7 in Type 1 diabetes Kerry A. McLaughlin1, Carolyn C. Richardson1,2, Aarthi Ravishankar1, Christina Brigatti3, Daniela Liberati4, Vito Lampasona4, Lorenzo Piemonti3, Diana Morgan5, Richard G. Feltbower5 and Michael R. Christie1,2 1Diabetes Research Group, Division of Diabetes & Nutritional Sciences, King’s College London, London, U.K. 2School of Life Sciences, University of Lincoln, Lincoln, U.K. 3Diabetes Research Institute, IRCCS San Raffaele Scientific Institute, Milan, Italy 4Division of Genetics and Cellular Biology, IRCCS San Raffaele Scientific Institute, Milan, Italy 5Division of Epidemiology & Biostatistics, School of Medicine, University of Leeds, Leeds, UK Corresponding author: Dr Michael R Christie, School of Life Sciences, Joseph Banks Laboratories, University of Lincoln, Lincoln LN6 7DL, United Kingdom Phone: +44 1522 837434 Email: [email protected] Word count of abstract: 199 Word count of main text: 3,998 Number of figures: 4. One Supplementary Table 1 Diabetes Publish Ahead of Print, published online March 7, 2016 Diabetes Page 2 of 35 ABSTRACT The presence of autoantibodies to multiple islet autoantigens confers high risk for development of Type 1 diabetes. Four major autoantigens are established (insulin, glutamate decarboxylase, IA-2, and zinc transporter-8), but the molecular identity of a fifth, a 38kDa membrane glycoprotein (Glima), is unknown. Glima antibodies have been detectable only by immunoprecipitation from extracts of radiolabeled islet or neuronal cells. We sought to identify Glima to enable efficient assay of these autoantibodies. Mouse brain and lung were shown to express Glima. -

Downloaded 18 July 2014 with a 1% False Discovery Rate (FDR)
UC Berkeley UC Berkeley Electronic Theses and Dissertations Title Chemical glycoproteomics for identification and discovery of glycoprotein alterations in human cancer Permalink https://escholarship.org/uc/item/0t47b9ws Author Spiciarich, David Publication Date 2017 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California Chemical glycoproteomics for identification and discovery of glycoprotein alterations in human cancer by David Spiciarich A dissertation submitted in partial satisfaction of the requirements for the degree Doctor of Philosophy in Chemistry in the Graduate Division of the University of California, Berkeley Committee in charge: Professor Carolyn R. Bertozzi, Co-Chair Professor David E. Wemmer, Co-Chair Professor Matthew B. Francis Professor Amy E. Herr Fall 2017 Chemical glycoproteomics for identification and discovery of glycoprotein alterations in human cancer © 2017 by David Spiciarich Abstract Chemical glycoproteomics for identification and discovery of glycoprotein alterations in human cancer by David Spiciarich Doctor of Philosophy in Chemistry University of California, Berkeley Professor Carolyn R. Bertozzi, Co-Chair Professor David E. Wemmer, Co-Chair Changes in glycosylation have long been appreciated to be part of the cancer phenotype; sialylated glycans are found at elevated levels on many types of cancer and have been implicated in disease progression. However, the specific glycoproteins that contribute to cell surface sialylation are not well characterized, specifically in bona fide human cancer. Metabolic and bioorthogonal labeling methods have previously enabled enrichment and identification of sialoglycoproteins from cultured cells and model organisms. The goal of this work was to develop technologies that can be used for detecting changes in glycoproteins in clinical models of human cancer. -

Contiguous Gene Deletion of Chromosome Xp in Three Families
Case Report iMedPub Journals Journal of Rare Disorders: Diagnosis & Therapy 2015 http://wwwimedpub.com ISSN 2380-7245 Vol. 1 No. 1:3 DOI: 10.21767/2380-7245.100003 Contiguous Gene Deletion of Shailly Jain-Ghai1,5, Stephanie Skinner1, Chromosome Xp in Three Families Jessica Hartley2,3, Encompassing OTC, RPGR and Stephanie Fox4, TSPAN7 Genes Daniela Buhas4, Cheryl Rockman- Greenberg2,3 and Alicia Chan1,5 Abstract Ornithine transcarbamylase deficiency (OTCD) is the most common urea cycle 1 Medical Genetics Clinic, Stollery disorder. The classic presentation in males is hyperammonemic encephalopathy Children's Hospital, Edmonton, Alberta, in the early neonatal period. Given the X-linked inheritance of OTCD, presentation Canada in females is highly variable. We present three families with different contiguous 2 Program in Genetics and Metabolism, Winnipeg Regional Health Authority gene deletions on chromosome Xp. Deletion ofRPGR , OTC and TSPAN7 is common and University of Manitoba, Winnipeg, to all three families in our series. These cases highlight the variable phenotype Manitoba, Canada in manifesting OTCD female carriers, the complexity of OTCD management and 3 Department of Biochemistry and complex issues surrounding the option of liver transplantation when multiple Medical Genetics, University of other genetic factors play a role. Manitoba, Winnipeg, Manitoba, Canada 4 Department of Medical Genetics, Keywords: Ornithine transcarbamylase; Ornithine transcarbamylase deficiency; Montreal Children’s Hospital, McGill Contiguous gene deletion; -

Supplementary Table 1 Double Treatment Vs Single Treatment
Supplementary table 1 Double treatment vs single treatment Probe ID Symbol Gene name P value Fold change TC0500007292.hg.1 NIM1K NIM1 serine/threonine protein kinase 1.05E-04 5.02 HTA2-neg-47424007_st NA NA 3.44E-03 4.11 HTA2-pos-3475282_st NA NA 3.30E-03 3.24 TC0X00007013.hg.1 MPC1L mitochondrial pyruvate carrier 1-like 5.22E-03 3.21 TC0200010447.hg.1 CASP8 caspase 8, apoptosis-related cysteine peptidase 3.54E-03 2.46 TC0400008390.hg.1 LRIT3 leucine-rich repeat, immunoglobulin-like and transmembrane domains 3 1.86E-03 2.41 TC1700011905.hg.1 DNAH17 dynein, axonemal, heavy chain 17 1.81E-04 2.40 TC0600012064.hg.1 GCM1 glial cells missing homolog 1 (Drosophila) 2.81E-03 2.39 TC0100015789.hg.1 POGZ Transcript Identified by AceView, Entrez Gene ID(s) 23126 3.64E-04 2.38 TC1300010039.hg.1 NEK5 NIMA-related kinase 5 3.39E-03 2.36 TC0900008222.hg.1 STX17 syntaxin 17 1.08E-03 2.29 TC1700012355.hg.1 KRBA2 KRAB-A domain containing 2 5.98E-03 2.28 HTA2-neg-47424044_st NA NA 5.94E-03 2.24 HTA2-neg-47424360_st NA NA 2.12E-03 2.22 TC0800010802.hg.1 C8orf89 chromosome 8 open reading frame 89 6.51E-04 2.20 TC1500010745.hg.1 POLR2M polymerase (RNA) II (DNA directed) polypeptide M 5.19E-03 2.20 TC1500007409.hg.1 GCNT3 glucosaminyl (N-acetyl) transferase 3, mucin type 6.48E-03 2.17 TC2200007132.hg.1 RFPL3 ret finger protein-like 3 5.91E-05 2.17 HTA2-neg-47424024_st NA NA 2.45E-03 2.16 TC0200010474.hg.1 KIAA2012 KIAA2012 5.20E-03 2.16 TC1100007216.hg.1 PRRG4 proline rich Gla (G-carboxyglutamic acid) 4 (transmembrane) 7.43E-03 2.15 TC0400012977.hg.1 SH3D19 -
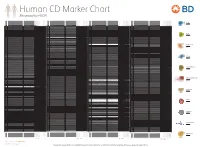
Reviewed by HLDA1
Human CD Marker Chart Reviewed by HLDA1 T Cell Key Markers CD3 CD4 CD Alternative Name Ligands & Associated Molecules T Cell B Cell Dendritic Cell NK Cell Stem Cell/Precursor Macrophage/Monocyte Granulocyte Platelet Erythrocyte Endothelial Cell Epithelial Cell CD Alternative Name Ligands & Associated Molecules T Cell B Cell Dendritic Cell NK Cell Stem Cell/Precursor Macrophage/Monocyte Granulocyte Platelet Erythrocyte Endothelial Cell Epithelial Cell CD Alternative Name Ligands & Associated Molecules T Cell B Cell Dendritic Cell NK Cell Stem Cell/Precursor Macrophage/Monocyte Granulocyte Platelet Erythrocyte Endothelial Cell Epithelial Cell CD Alternative Name Ligands & Associated Molecules T Cell B Cell Dendritic Cell NK Cell Stem Cell/Precursor Macrophage/Monocyte Granulocyte Platelet Erythrocyte Endothelial Cell Epithelial Cell CD8 CD1a R4, T6, Leu6, HTA1 b-2-Microglobulin, CD74 + + + – + – – – CD74 DHLAG, HLADG, Ia-g, li, invariant chain HLA-DR, CD44 + + + + + + CD158g KIR2DS5 + + CD248 TEM1, Endosialin, CD164L1, MGC119478, MGC119479 Collagen I/IV Fibronectin + ST6GAL1, MGC48859, SIAT1, ST6GALL, ST6N, ST6 b-Galactosamide a-2,6-sialyl- CD1b R1, T6m Leu6 b-2-Microglobulin + + + – + – – – CD75 CD22 CD158h KIR2DS1, p50.1 HLA-C + + CD249 APA, gp160, EAP, ENPEP + + tranferase, Sialo-masked lactosamine, Carbohydrate of a2,6 sialyltransferase + – – + – – + – – CD1c M241, R7, T6, Leu6, BDCA1 b-2-Microglobulin + + + – + – – – CD75S a2,6 Sialylated lactosamine CD22 (proposed) + + – – + + – + + + CD158i KIR2DS4, p50.3 HLA-C + – + CD252 TNFSF4, -

Native Nanodiscs and the Convergence of Lipidomics, Metabolomics, Interactomics and Proteomics
applied sciences Review Native Nanodiscs and the Convergence of Lipidomics, Metabolomics, Interactomics and Proteomics Michael Overduin * and Mansoore Esmaili Department of Biochemistry, University of Alberta, Edmonton, AB T6G 2H7, Canada; [email protected] * Correspondence: [email protected]; Tel.: +1-780-492-3518 Received: 1 March 2019; Accepted: 19 March 2019; Published: 24 March 2019 Featured Application: Nanodiscs formed by styrene maleic acid copolymers allow separation of biologically intact protein complexes with native ligands for syst-OMICS analysis. Abstract: The omics disciplines remain largely distinct sciences due to the necessity of separating molecular classes for different assays. For example, water-soluble and lipid bilayer-bound proteins and metabolites are usually studied separately. Nonetheless, it is at the interface between these sciences where biology happens. That is, lipid-interacting proteins typically recognize and transduce signals and regulate the flow of metabolites in the cell. Technologies are emerging to converge the omics. It is now possible to separate intact membrane:protein assemblies (memteins) directly from intact cells or cell membranes. Such complexes mediate complete metabolon, receptor, channel, and transporter functions. The use of poly(styrene-co-maleic acid) (SMA) copolymers has allowed their separation in a single step without any exposure to synthetic detergents or artificial lipids. This is a critical development as these agents typically strip away biological lipids, signals, and metabolites from their physiologically-relevant positions on proteins. The resulting SMA lipid particles (SMALPs) represent native nanodiscs that are suitable for elucidation of structures and interactions that occur in vivo. Compatible tools for resolving the contained memteins include X-ray diffraction (XRD), cryo-electron microscopy (cryoEM), mass spectrometry (MS), and nuclear magnetic resonance (NMR) spectroscopy.