Downloaded 18 July 2014 with a 1% False Discovery Rate (FDR)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

And 0-Linked Oligosaccharides of the HLA-DR-Associated Invariant Chain
Proc. Nati. Acad. Sci. USA Vol. 81, pp. 1287-1291, March 1984 Biochemistry Monensin prevents terminal glycosylation of the N- and 0-linked oligosaccharides of the HLA-DR-associated invariant chain and inhibits its dissociation from the a-,8 chain complex (carboxylic ionophores/class II andgens/post-translational processing) CAROLYN E. MACHAMER AND PETER CRESSWELL Department of Microbiology and Immunology, Duke University Medical Center, Durham, NC 27710 Communicated by D. Bernard Amos, October 24, 1983 ABSTRACT In B-lymphoblastoid cells, the HLA-DR-as- endocytosis is most likely a result of the increased pH inside sociated invariant chain is processed to a form containing 0- prelysosomal endosomes observed in monensin-treated cells linked as well as N-linked oligosaccharides. After neuraminidase (15). treatment, the O-linked carbohydrate is susceptible to diges- We previously described post-translational modifications tion with an endoglycosidase (endo-fi-N-acetylgalactosamini- of the N-linked glycan units of the invariant chain and sug- dase) that cleaves glycans with the structure Gal(l-+3)- gested that 0-linked carbohydrate was-added during its tran- GaINAc-Ser/Thr, and sialic acid can be added back to this sient association with HLA-DR antigens (5). Here we prove core oligosaccharide by specific sialyltransferases. Treatment the addition of 0-linked glycans and report the effects of of cells with the sodium ionophore monensin markedly affects monensin on the post-translational processing of the invari- the post-translational processing of the invariant chain, al- ant chain and on its interaction with HLA-DR antigens in B- though that of associated a and ,3 chains is minimally affected. -

Upregulation of Peroxisome Proliferator-Activated Receptor-Α And
Upregulation of peroxisome proliferator-activated receptor-α and the lipid metabolism pathway promotes carcinogenesis of ampullary cancer Chih-Yang Wang, Ying-Jui Chao, Yi-Ling Chen, Tzu-Wen Wang, Nam Nhut Phan, Hui-Ping Hsu, Yan-Shen Shan, Ming-Derg Lai 1 Supplementary Table 1. Demographics and clinical outcomes of five patients with ampullary cancer Time of Tumor Time to Age Differentia survival/ Sex Staging size Morphology Recurrence recurrence Condition (years) tion expired (cm) (months) (months) T2N0, 51 F 211 Polypoid Unknown No -- Survived 193 stage Ib T2N0, 2.41.5 58 F Mixed Good Yes 14 Expired 17 stage Ib 0.6 T3N0, 4.53.5 68 M Polypoid Good No -- Survived 162 stage IIA 1.2 T3N0, 66 M 110.8 Ulcerative Good Yes 64 Expired 227 stage IIA T3N0, 60 M 21.81 Mixed Moderate Yes 5.6 Expired 16.7 stage IIA 2 Supplementary Table 2. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis of an ampullary cancer microarray using the Database for Annotation, Visualization and Integrated Discovery (DAVID). This table contains only pathways with p values that ranged 0.0001~0.05. KEGG Pathway p value Genes Pentose and 1.50E-04 UGT1A6, CRYL1, UGT1A8, AKR1B1, UGT2B11, UGT2A3, glucuronate UGT2B10, UGT2B7, XYLB interconversions Drug metabolism 1.63E-04 CYP3A4, XDH, UGT1A6, CYP3A5, CES2, CYP3A7, UGT1A8, NAT2, UGT2B11, DPYD, UGT2A3, UGT2B10, UGT2B7 Maturity-onset 2.43E-04 HNF1A, HNF4A, SLC2A2, PKLR, NEUROD1, HNF4G, diabetes of the PDX1, NR5A2, NKX2-2 young Starch and sucrose 6.03E-04 GBA3, UGT1A6, G6PC, UGT1A8, ENPP3, MGAM, SI, metabolism -

The Role of Protein Crystallography in Defining the Mechanisms of Biogenesis and Catalysis in Copper Amine Oxidase
Int. J. Mol. Sci. 2012, 13, 5375-5405; doi:10.3390/ijms13055375 OPEN ACCESS International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Review The Role of Protein Crystallography in Defining the Mechanisms of Biogenesis and Catalysis in Copper Amine Oxidase Valerie J. Klema and Carrie M. Wilmot * Department of Biochemistry, Molecular Biology, and Biophysics, University of Minnesota, 321 Church St. SE, Minneapolis, MN 55455, USA; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +1-612-624-2406; Fax: +1-612-624-5121. Received: 6 April 2012; in revised form: 22 April 2012 / Accepted: 26 April 2012 / Published: 3 May 2012 Abstract: Copper amine oxidases (CAOs) are a ubiquitous group of enzymes that catalyze the conversion of primary amines to aldehydes coupled to the reduction of O2 to H2O2. These enzymes utilize a wide range of substrates from methylamine to polypeptides. Changes in CAO activity are correlated with a variety of human diseases, including diabetes mellitus, Alzheimer’s disease, and inflammatory disorders. CAOs contain a cofactor, 2,4,5-trihydroxyphenylalanine quinone (TPQ), that is required for catalytic activity and synthesized through the post-translational modification of a tyrosine residue within the CAO polypeptide. TPQ generation is a self-processing event only requiring the addition of oxygen and Cu(II) to the apoCAO. Thus, the CAO active site supports two very different reactions: TPQ synthesis, and the two electron oxidation of primary amines. Crystal structures are available from bacterial through to human sources, and have given insight into substrate preference, stereospecificity, and structural changes during biogenesis and catalysis. -
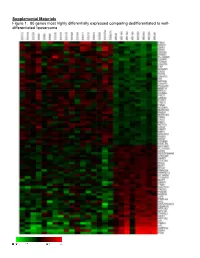
Supplemental Materials Figure 1. 80 Genes Most Highly Differentially Expressed Comparing Dedifferentiated to Well- Differentiated Liposarcoma
Supplemental Materials Figure 1. 80 genes most highly differentially expressed comparing dedifferentiated to well- differentiated liposarcoma Figure 2. Validation of microa CDC2, RACGAP1, FGFR-2, MAD2 and CITED1 200 CDK4 by Liposarcoma Subtype 16 0 12 0 80 Expression40 Level 0 Nl Fat rray data by quantitative RT-P Well Diff 16 0 p16 by Liposarcoma Subtype Dediff 12 0 80 Myxoid Expression40 Level Round Cell 0 12 0 MDM2 by Liposarcoma Subtype 10 0 Pleomorphic Nl Fat 80 60 40 Expression Level 20 RACGAP1 by LiposarcomaWell Diff Subtype 0 70 CR for genes CDK4, MDM2, p16, 60 Dediff 50 Nl Fat 40 30 Myxoid Expression20 Level Well Diff 10 50 0 Round Cell CDC2 by Liposarcoma Subtype Dediff 40 Nl Fat Pleomorphic 30 Myxoid 20 Expression Level Well Diff 10 MAD2L1 by Liposarcoma Subtype Round Cell 60 0 50 Dediff Pleomorphic 40 Nl Fat 30 Myxoid 20 Expression Level Well Diff 10 FGFR-2 by Liposarcoma Subtype 0 Round Cell 200 16 0 Dediff Nl Fat Pleomorphic 12 0 Myxoid 80 Expression Level Well Diff 40 Round Cell 0 Dediff Pleomorphic Nl Fat Myxoid Well Diff CITED1 by Liposarcoma Subtype Round Cell 10 0 80 Dediff Pleomorphic 60 40 Myxoid Expression Level 20 0 Round Cell Nl Fat Pleomorphic Well Diff Dediff Myxoid Round Cell Pleomorphic Table 1. 142 Gene classifier for liposarcoma subtype The genes used for each pair wise subtype comparison are grouped together. The flag column indicates which genes are unique to each subtype comparison. The values show the mean expression levels (actually the mean of the log expression levels was computed and than transformed back to absolute expression level). -

Stelios Pavlidis3, Matthew Loza3, Fred Baribaud3, Anthony
Supplementary Data Th2 and non-Th2 molecular phenotypes of asthma using sputum transcriptomics in UBIOPRED Chih-Hsi Scott Kuo1.2, Stelios Pavlidis3, Matthew Loza3, Fred Baribaud3, Anthony Rowe3, Iaonnis Pandis2, Ana Sousa4, Julie Corfield5, Ratko Djukanovic6, Rene 7 7 8 2 1† Lutter , Peter J. Sterk , Charles Auffray , Yike Guo , Ian M. Adcock & Kian Fan 1†* # Chung on behalf of the U-BIOPRED consortium project team 1Airways Disease, National Heart & Lung Institute, Imperial College London, & Biomedical Research Unit, Biomedical Research Unit, Royal Brompton & Harefield NHS Trust, London, United Kingdom; 2Department of Computing & Data Science Institute, Imperial College London, United Kingdom; 3Janssen Research and Development, High Wycombe, Buckinghamshire, United Kingdom; 4Respiratory Therapeutic Unit, GSK, Stockley Park, United Kingdom; 5AstraZeneca R&D Molndal, Sweden and Areteva R&D, Nottingham, United Kingdom; 6Faculty of Medicine, Southampton University, Southampton, United Kingdom; 7Faculty of Medicine, University of Amsterdam, Amsterdam, Netherlands; 8European Institute for Systems Biology and Medicine, CNRS-ENS-UCBL, Université de Lyon, France. †Contributed equally #Consortium project team members are listed under Supplementary 1 Materials *To whom correspondence should be addressed: [email protected] 2 List of the U-BIOPRED Consortium project team members Uruj Hoda & Christos Rossios, Airways Disease, National Heart & Lung Institute, Imperial College London, UK & Biomedical Research Unit, Biomedical Research Unit, Royal -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

New PDF Document
888.267.4436 [email protected] www.origene.com Name:CLDN6 mouse monoclonal antibody, clone OTI3E3 (formerly 3E3) Catalog: TA507011 Product Data Sheet - TRUEMAB Components: • CLDN6 mouse monoclonal antibody, clone OTI3E3 (formerly 3E3) (TA507011) • WB positive control also available: 20ug myc-DDK tagged CLDN6 HEK293T over-expression lysate lyophilized in RIPA buffer (LC412034). (Reconstitute into 20ul of 1x SDS sample buffer before loading; load 5ul per lane as WB control or as desired) Amount: 100ul Immunogen: Full length human recombinant protein of human CLDN6(NP_067018) produced in HEK293T cell. Host: Mouse Isotype: IgG1 Species Reactivity: Human Guaranteed WB, IF Applications: Suggested WB 1:1000, IF 1:100, Dilutions: Concentration: 1 mg/ml Buffer: PBS (PH 7.3) containing 1% BSA, 50% glycerol and 0.02% sodium azide. Purification: Purified from mouse ascites fluids by affinity chromatography Storage Condition: Shipped at -20C or with ice packs. Upon delivery store at -20C. Dilute in PBS (pH7.3) if necessary. Stable for 12 months from date of receipt. Avoid repeated freeze-thaws. Target Target Name: Homo sapiens claudin 6 (CLDN6) Alternative Name: OTTHUMP00000159248; claudin 6 Database Link: NP_067018 Entrez Gene 9074 Human Function: Tight junctions represent one mode of cell-to-cell adhesion in epithelial or endothelial cell sheets, forming continuous seals around cells and serving as a physical barrier to prevent solutes and water from passing freely through the paracellular space. These junctions are comprised of sets of continuous networking strands in the outwardly facing cytoplasmic leaflet, with complementary grooves in the inwardly facing extracytoplasmic leaflet. This gene encodes a component of tight junction strands, which This product is to be used for laboratory only. -

Protein Identities in Evs Isolated from U87-MG GBM Cells As Determined by NG LC-MS/MS
Protein identities in EVs isolated from U87-MG GBM cells as determined by NG LC-MS/MS. No. Accession Description Σ Coverage Σ# Proteins Σ# Unique Peptides Σ# Peptides Σ# PSMs # AAs MW [kDa] calc. pI 1 A8MS94 Putative golgin subfamily A member 2-like protein 5 OS=Homo sapiens PE=5 SV=2 - [GG2L5_HUMAN] 100 1 1 7 88 110 12,03704523 5,681152344 2 P60660 Myosin light polypeptide 6 OS=Homo sapiens GN=MYL6 PE=1 SV=2 - [MYL6_HUMAN] 100 3 5 17 173 151 16,91913397 4,652832031 3 Q6ZYL4 General transcription factor IIH subunit 5 OS=Homo sapiens GN=GTF2H5 PE=1 SV=1 - [TF2H5_HUMAN] 98,59 1 1 4 13 71 8,048185945 4,652832031 4 P60709 Actin, cytoplasmic 1 OS=Homo sapiens GN=ACTB PE=1 SV=1 - [ACTB_HUMAN] 97,6 5 5 35 917 375 41,70973209 5,478027344 5 P13489 Ribonuclease inhibitor OS=Homo sapiens GN=RNH1 PE=1 SV=2 - [RINI_HUMAN] 96,75 1 12 37 173 461 49,94108966 4,817871094 6 P09382 Galectin-1 OS=Homo sapiens GN=LGALS1 PE=1 SV=2 - [LEG1_HUMAN] 96,3 1 7 14 283 135 14,70620005 5,503417969 7 P60174 Triosephosphate isomerase OS=Homo sapiens GN=TPI1 PE=1 SV=3 - [TPIS_HUMAN] 95,1 3 16 25 375 286 30,77169764 5,922363281 8 P04406 Glyceraldehyde-3-phosphate dehydrogenase OS=Homo sapiens GN=GAPDH PE=1 SV=3 - [G3P_HUMAN] 94,63 2 13 31 509 335 36,03039959 8,455566406 9 Q15185 Prostaglandin E synthase 3 OS=Homo sapiens GN=PTGES3 PE=1 SV=1 - [TEBP_HUMAN] 93,13 1 5 12 74 160 18,68541938 4,538574219 10 P09417 Dihydropteridine reductase OS=Homo sapiens GN=QDPR PE=1 SV=2 - [DHPR_HUMAN] 93,03 1 1 17 69 244 25,77302971 7,371582031 11 P01911 HLA class II histocompatibility antigen, -

Studies on the Proteome of Human Hair - Identifcation of Histones and Deamidated Keratins Received: 15 August 2017 Sunil S
www.nature.com/scientificreports OPEN Studies on the Proteome of Human Hair - Identifcation of Histones and Deamidated Keratins Received: 15 August 2017 Sunil S. Adav 1, Roopa S. Subbaiaih2, Swat Kim Kerk 2, Amelia Yilin Lee 2,3, Hui Ying Lai3,4, Accepted: 12 January 2018 Kee Woei Ng3,4,7, Siu Kwan Sze 1 & Artur Schmidtchen2,5,6 Published: xx xx xxxx Human hair is laminar-fbrous tissue and an evolutionarily old keratinization product of follicle trichocytes. Studies on the hair proteome can give new insights into hair function and lead to the development of novel biomarkers for hair in health and disease. Human hair proteins were extracted by detergent and detergent-free techniques. We adopted a shotgun proteomics approach, which demonstrated a large extractability and variety of hair proteins after detergent extraction. We found an enrichment of keratin, keratin-associated proteins (KAPs), and intermediate flament proteins, which were part of protein networks associated with response to stress, innate immunity, epidermis development, and the hair cycle. Our analysis also revealed a signifcant deamidation of keratin type I and II, and KAPs. The hair shafts were found to contain several types of histones, which are well known to exert antimicrobial activity. Analysis of the hair proteome, particularly its composition, protein abundances, deamidated hair proteins, and modifcation sites, may ofer a novel approach to explore potential biomarkers of hair health quality, hair diseases, and aging. Hair is an important and evolutionarily conserved structure. It originates from hair follicles deep within the der- mis and is mainly composed of hair keratins and KAPs, which form a complex network that contributes to the rigidity and mechanical properties. -

The Role of Genetic Variation in Predisposition to Alcohol-Related Chronic Pancreatitis
The Role of Genetic Variation in Predisposition to Alcohol-related Chronic Pancreatitis Thesis submitted in accordance with the requirements of the University of Liverpool for the degree of Doctor in Philosophy by Marianne Lucy Johnstone April 2015 The Role of Genetic Variation in Predisposition to Alcohol-related Chronic Pancreatitis 2015 Abstract Background Chronic pancreatitis (CP) is a disease of fibrosis of the pancreas for which alcohol is the main causative agent. However, only a small proportion of alcoholics develop chronic pancreatitis. Genetic polymorphism may affect pancreatitis risk. Aim To determine the factors required to classify a chronic pancreatic population and identify genetic variations that may explain why only some alcoholics develop chronic pancreatitis. Methods The most appropriate method of diagnosing CP was assessed using a systematic review. Genetics of different populations of alcohol-related chronic pancreatitics (ACP) were explored using four different techniques: genome-wide association study (GWAS); custom arrays; PCR of variable nucleotide tandem repeats (VNTR) and next generation sequencing (NGS) of selected genes. Results EUS and sMR were identified as giving the overall best sensitivity and specificity for diagnosing CP. GWAS revealed two associations with CP (identified and replicated) at PRSS1-PRSS2_rs10273639 (OR 0.73, 95% CI 0.68-0.79) and X-linked CLDN2_rs12688220 (OR 1.39, 1.28-1.49) and the association was more pronounced in the ACP group (OR 0.56, 0.48-0.64)and OR 2.11, 1.84-2.42). The previously identified VNTR in CEL was shown to have a lower frequency of the normal repeat in ACP than alcoholic liver disease (ALD; OR 0.61, 0.41-0.93). -

Atrazine and Cell Death Symbol Synonym(S)
Supplementary Table S1: Atrazine and Cell Death Symbol Synonym(s) Entrez Gene Name Location Family AR AIS, Andr, androgen receptor androgen receptor Nucleus ligand- dependent nuclear receptor atrazine 1,3,5-triazine-2,4-diamine Other chemical toxicant beta-estradiol (8R,9S,13S,14S,17S)-13-methyl- Other chemical - 6,7,8,9,11,12,14,15,16,17- endogenous decahydrocyclopenta[a]phenanthrene- mammalian 3,17-diol CGB (includes beta HCG5, CGB3, CGB5, CGB7, chorionic gonadotropin, beta Extracellular other others) CGB8, chorionic gonadotropin polypeptide Space CLEC11A AW457320, C-type lectin domain C-type lectin domain family 11, Extracellular growth factor family 11, member A, STEM CELL member A Space GROWTH FACTOR CYP11A1 CHOLESTEROL SIDE-CHAIN cytochrome P450, family 11, Cytoplasm enzyme CLEAVAGE ENZYME subfamily A, polypeptide 1 CYP19A1 Ar, ArKO, ARO, ARO1, Aromatase cytochrome P450, family 19, Cytoplasm enzyme subfamily A, polypeptide 1 ESR1 AA420328, Alpha estrogen receptor,(α) estrogen receptor 1 Nucleus ligand- dependent nuclear receptor estrogen C18 steroids, oestrogen Other chemical drug estrogen receptor ER, ESR, ESR1/2, esr1/esr2 Nucleus group estrone (8R,9S,13S,14S)-3-hydroxy-13-methyl- Other chemical - 7,8,9,11,12,14,15,16-octahydro-6H- endogenous cyclopenta[a]phenanthren-17-one mammalian G6PD BOS 25472, G28A, G6PD1, G6PDX, glucose-6-phosphate Cytoplasm enzyme Glucose-6-P Dehydrogenase dehydrogenase GATA4 ASD2, GATA binding protein 4, GATA binding protein 4 Nucleus transcription TACHD, TOF, VSD1 regulator GHRHR growth hormone releasing -

Α Are Regulated by Heat Shock Protein 90
The Levels of Retinoic Acid-Inducible Gene I Are Regulated by Heat Shock Protein 90- α Tomoh Matsumiya, Tadaatsu Imaizumi, Hidemi Yoshida, Kei Satoh, Matthew K. Topham and Diana M. Stafforini This information is current as of October 2, 2021. J Immunol 2009; 182:2717-2725; ; doi: 10.4049/jimmunol.0802933 http://www.jimmunol.org/content/182/5/2717 Downloaded from References This article cites 44 articles, 19 of which you can access for free at: http://www.jimmunol.org/content/182/5/2717.full#ref-list-1 Why The JI? Submit online. http://www.jimmunol.org/ • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average by guest on October 2, 2021 Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2009 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology The Levels of Retinoic Acid-Inducible Gene I Are Regulated by Heat Shock Protein 90-␣1 Tomoh Matsumiya,*‡ Tadaatsu Imaizumi,‡ Hidemi Yoshida,‡ Kei Satoh,‡ Matthew K. Topham,*† and Diana M. Stafforini2*† Retinoic acid-inducible gene I (RIG-I) is an intracellular pattern recognition receptor that plays important roles during innate immune responses to viral dsRNAs.