Diversity of Radiolarian Families Through Time
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Molecular Phylogenetic Position of Hexacontium Pachydermum Jørgensen (Radiolaria)
Marine Micropaleontology 73 (2009) 129–134 Contents lists available at ScienceDirect Marine Micropaleontology journal homepage: www.elsevier.com/locate/marmicro Molecular phylogenetic position of Hexacontium pachydermum Jørgensen (Radiolaria) Tomoko Yuasa a,⁎, Jane K. Dolven b, Kjell R. Bjørklund b, Shigeki Mayama c, Osamu Takahashi a a Department of Astronomy and Earth Sciences, Tokyo Gakugei University, Koganei, Tokyo 184-8501, Japan b Natural History Museum, University of Oslo, P.O. Box 1172, Blindern, 0318 Oslo, Norway c Department of Biology, Tokyo Gakugei University, Koganei, Tokyo 184-8501, Japan article info abstract Article history: The taxonomic affiliation of Hexacontium pachydermum Jørgensen, specifically whether it belongs to the Received 9 April 2009 order Spumellarida or the order Entactinarida, is a subject of ongoing debate. In this study, we sequenced the Received in revised form 3 August 2009 18S rRNA gene of H. pachydermum and of three spherical spumellarians of Cladococcus viminalis Haeckel, Accepted 7 August 2009 Arachnosphaera myriacantha Haeckel, and Astrosphaera hexagonalis Haeckel. Our molecular phylogenetic analysis revealed that the spumellarian species of C. viminalis, A. myriacantha, and A. hexagonalis form a Keywords: monophyletic group. Moreover, this clade occupies a sister position to the clade comprising the spongodiscid Radiolaria fi Entactinarida spumellarians, coccodiscid spumellarians, and H. pachydermum. This nding is contrary to the results of Spumellarida morphological studies based on internal spicular morphology, placing H. pachydermum in the order Nassellarida Entactinarida, which had been considered to have a common ancestor shared with the nassellarians. 18S rRNA gene © 2009 Elsevier B.V. All rights reserved. Molecular phylogeny. 1. Introduction the order Entactinarida has an inner spicular system homologenous with that of the order Nassellarida. -

Anoxygenic Photosynthesis in Photolithotrophic Sulfur Bacteria and Their Role in Detoxication of Hydrogen Sulfide
antioxidants Review Anoxygenic Photosynthesis in Photolithotrophic Sulfur Bacteria and Their Role in Detoxication of Hydrogen Sulfide Ivan Kushkevych 1,* , Veronika Bosáková 1,2 , Monika Vítˇezová 1 and Simon K.-M. R. Rittmann 3,* 1 Department of Experimental Biology, Faculty of Science, Masaryk University, 62500 Brno, Czech Republic; [email protected] (V.B.); [email protected] (M.V.) 2 Department of Biology, Faculty of Medicine, Masaryk University, 62500 Brno, Czech Republic 3 Archaea Physiology & Biotechnology Group, Department of Functional and Evolutionary Ecology, Universität Wien, 1090 Vienna, Austria * Correspondence: [email protected] (I.K.); [email protected] (S.K.-M.R.R.); Tel.: +420-549-495-315 (I.K.); +431-427-776-513 (S.K.-M.R.R.) Abstract: Hydrogen sulfide is a toxic compound that can affect various groups of water microorgan- isms. Photolithotrophic sulfur bacteria including Chromatiaceae and Chlorobiaceae are able to convert inorganic substrate (hydrogen sulfide and carbon dioxide) into organic matter deriving energy from photosynthesis. This process takes place in the absence of molecular oxygen and is referred to as anoxygenic photosynthesis, in which exogenous electron donors are needed. These donors may be reduced sulfur compounds such as hydrogen sulfide. This paper deals with the description of this metabolic process, representatives of the above-mentioned families, and discusses the possibility using anoxygenic phototrophic microorganisms for the detoxification of toxic hydrogen sulfide. Moreover, their general characteristics, morphology, metabolism, and taxonomy are described as Citation: Kushkevych, I.; Bosáková, well as the conditions for isolation and cultivation of these microorganisms will be presented. V.; Vítˇezová,M.; Rittmann, S.K.-M.R. -

Limits of Life on Earth Some Archaea and Bacteria
Limits of life on Earth Thermophiles Temperatures up to ~55C are common, but T > 55C is Some archaea and bacteria (extremophiles) can live in associated usually with geothermal features (hot springs, environments that we would consider inhospitable to volcanic activity etc) life (heat, cold, acidity, high pressure etc) Thermophiles are organisms that can successfully live Distinguish between growth and survival: many organisms can survive intervals of harsh conditions but could not at high temperatures live permanently in such conditions (e.g. seeds, spores) Best studied extremophiles: may be relevant to the Interest: origin of life. Very hot environments tolerable for life do not seem to exist elsewhere in the Solar System • analogs for extraterrestrial environments • `extreme’ conditions may have been more common on the early Earth - origin of life? • some unusual environments (e.g. subterranean) are very widespread Extraterrestrial Life: Spring 2008 Extraterrestrial Life: Spring 2008 Grand Prismatic Spring, Yellowstone National Park Hydrothermal vents: high pressure in the deep ocean allows liquid water Colors on the edge of the at T >> 100C spring are caused by different colonies of thermophilic Vents emit superheated water (300C or cyanobacteria and algae more) that is rich in minerals Hottest water is lifeless, but `cooler’ ~50 species of such thermophiles - mostly archae with some margins support array of thermophiles: cyanobacteria and anaerobic photosynthetic bacteria oxidize sulphur, manganese, grow on methane + carbon monoxide etc… Sulfolobus: optimum T ~ 80C, minimum 60C, maximum 90C, also prefer a moderately acidic pH. Live by oxidizing sulfur Known examples can grow (i.e. multiply) at temperatures which is abundant near hot springs. -

Rhizaria, Cercozoa)
Protist, Vol. 166, 363–373, July 2015 http://www.elsevier.de/protis Published online date 28 May 2015 ORIGINAL PAPER Molecular Phylogeny of the Widely Distributed Marine Protists, Phaeodaria (Rhizaria, Cercozoa) a,1 a a b Yasuhide Nakamura , Ichiro Imai , Atsushi Yamaguchi , Akihiro Tuji , c d Fabrice Not , and Noritoshi Suzuki a Plankton Laboratory, Graduate School of Fisheries Sciences, Hokkaido University, Hakodate, Hokkaido 041–8611, Japan b Department of Botany, National Museum of Nature and Science, Tsukuba 305–0005, Japan c CNRS, UMR 7144 & Université Pierre et Marie Curie, Station Biologique de Roscoff, Equipe EPPO - Evolution du Plancton et PaléoOcéans, Place Georges Teissier, 29682 Roscoff, France d Institute of Geology and Paleontology, Graduate School of Science, Tohoku University, Sendai 980–8578, Japan Submitted January 1, 2015; Accepted May 19, 2015 Monitoring Editor: David Moreira Phaeodarians are a group of widely distributed marine cercozoans. These plankton organisms can exhibit a large biomass in the environment and are supposed to play an important role in marine ecosystems and in material cycles in the ocean. Accurate knowledge of phaeodarian classification is thus necessary to better understand marine biology, however, phylogenetic information on Phaeodaria is limited. The present study analyzed 18S rDNA sequences encompassing all existing phaeodarian orders, to clarify their phylogenetic relationships and improve their taxonomic classification. The mono- phyly of Phaeodaria was confirmed and strongly supported by phylogenetic analysis with a larger data set than in previous studies. The phaeodarian clade contained 11 subclades which generally did not correspond to the families and orders of the current classification system. Two families (Challengeri- idae and Aulosphaeridae) and two orders (Phaeogromida and Phaeocalpida) are possibly polyphyletic or paraphyletic, and consequently the classification needs to be revised at both the family and order levels by integrative taxonomy approaches. -

PROTISTS Shore and the Waves Are Large, Often the Largest of a Storm Event, and with a Long Period
(seas), and these waves can mobilize boulders. During this phase of the storm the rapid changes in current direction caused by these large, short-period waves generate high accelerative forces, and it is these forces that ultimately can move even large boulders. Traditionally, most rocky-intertidal ecological stud- ies have been conducted on rocky platforms where the substrate is composed of stable basement rock. Projec- tiles tend to be uncommon in these types of habitats, and damage from projectiles is usually light. Perhaps for this reason the role of projectiles in intertidal ecology has received little attention. Boulder-fi eld intertidal zones are as common as, if not more common than, rock plat- forms. In boulder fi elds, projectiles are abundant, and the evidence of damage due to projectiles is obvious. Here projectiles may be one of the most important defi ning physical forces in the habitat. SEE ALSO THE FOLLOWING ARTICLES Geology, Coastal / Habitat Alteration / Hydrodynamic Forces / Wave Exposure FURTHER READING Carstens. T. 1968. Wave forces on boundaries and submerged bodies. Sarsia FIGURE 6 The intertidal zone on the north side of Cape Blanco, 34: 37–60. Oregon. The large, smooth boulders are made of serpentine, while Dayton, P. K. 1971. Competition, disturbance, and community organi- the surrounding rock from which the intertidal platform is formed zation: the provision and subsequent utilization of space in a rocky is sandstone. The smooth boulders are from a source outside the intertidal community. Ecological Monographs 45: 137–159. intertidal zone and were carried into the intertidal zone by waves. Levin, S. A., and R. -

September 2002
RADI LARIA VOLUME 20 SEPTEMBER 2002 NEWSLETTER OF THE INTERNATIONAL ASSOCIATION OF RADIOLARIAN PALEONTOLOGISTS ISSN: 0297.5270 INTERRAD International Association of Radiolarian Paleontologists A Research Group of the International Paleontological Association Officers of the Association President Past President PETER BAUMBARTNER JOYCE R. BLUEFORD Lausanne, Switzerland California, USA [email protected] [email protected] Secretary Treasurer JONATHAN AITCHISON ELSPETH URQUHART Department of Earth Sciences JOIDES Office University of Hong Kong Department of Geology and Geophysics Pokfulam Road, University of Miami - RSMAS Hong Kong SAR, 4600 Rickenbacker Causeway CHINA Miami FL 33149 Florida Tel: (852) 2859 8047 Fax: (852) 2517 6912 U.S.A. e-mail: [email protected] Tel: 1-305-361-4668 Fax: 1-305-361-4632 Email: [email protected] Working Group Chairmen Paleozoic Cenozoic PATRICIA, WHALEN, U.S.A. ANNIKA SANFILIPPO California, U.S.A. [email protected] [email protected] Mesozoic Recent RIE S. HORI Matsuyama, JAPAN DEMETRIO BOLTOVSKOY Buenos Aires, ARGENTINA [email protected] [email protected] INTERRAD is an international non-profit organization for researchers interested in all aspects of radiolarian taxonomy, palaeobiology, morphology, biostratigraphy, biology, ecology and paleoecology. INTERRAD is a Research Group of the International Paleontological Association (IPA). Since 1978 members of INTERRAD meet every three years to present papers and exchange ideas and materials INTERRAD MEMBERSHIP: The international Association of Radiolarian Paleontologists is open to any one interested on receipt of subscription. The actual fee US $ 15 per year. Membership queries and subscription send to Treasurer. Changes of address can be sent to the Secretary. -

Radiozoa (Acantharia, Phaeodaria and Radiolaria) and Heliozoa
MICC16 26/09/2005 12:21 PM Page 188 CHAPTER 16 Radiozoa (Acantharia, Phaeodaria and Radiolaria) and Heliozoa Cavalier-Smith (1987) created the phylum Radiozoa to Radiating outwards from the central capsule are the include the marine zooplankton Acantharia, Phaeodaria pseudopodia, either as thread-like filopodia or as and Radiolaria, united by the presence of a central axopodia, which have a central rod of fibres for rigid- capsule. Only the Radiolaria including the siliceous ity. The ectoplasm typically contains a zone of frothy, Polycystina (which includes the orders Spumellaria gelatinous bubbles, collectively termed the calymma and Nassellaria) and the mixed silica–organic matter and a swarm of yellow symbiotic algae called zooxan- Phaeodaria are preserved in the fossil record. The thellae. The calymma in some spumellarian Radiolaria Acantharia have a skeleton of strontium sulphate can be so extensive as to obscure the skeleton. (i.e. celestine SrSO4). The radiolarians range from the A mineralized skeleton is usually present within the Cambrian and have a virtually global, geographical cell and comprises, in the simplest forms, either radial distribution and a depth range from the photic zone or tangential elements, or both. The radial elements down to the abyssal plains. Radiolarians are most useful consist of loose spicules, external spines or internal for biostratigraphy of Mesozoic and Cenozoic deep sea bars. They may be hollow or solid and serve mainly to sediments and as palaeo-oceanographical indicators. support the axopodia. The tangential elements, where Heliozoa are free-floating protists with roughly present, generally form a porous lattice shell of very spherical shells and thread-like pseudopodia that variable morphology, such as spheres, spindles and extend radially over a delicate silica endoskeleton. -

Algae and Cyanobacteria in Coastal and Estuarine Waters
CHAPTER 7 Algae and cyanobacteria in coastal and estuarine waters n coastal and estuarine waters, algae range from single-celled forms to the seaweeds. ICyanobacteria are organisms with some characteristics of bacteria and some of algae. They are similar in size to the unicellular algae and, unlike other bacteria, contain blue-green or green pigments and are able to perform photosynthesis; thus, they are also termed blue-green algae. Algal blooms in the sea have occurred throughout recorded history but have been increasing during recent decades (Anderson, 1989; Smayda, 1989a; Hallegraeff, 1993). In several areas (e.g., the Baltic and North seas, the Adriatic Sea, Japanese coastal waters and the Gulf of Mexico), algal blooms are a recurring phe- nomenon. The increased frequency of occurrence has accompanied nutrient enrich- ment of coastal waters on a global scale (Smayda, 1989b). Blooms of non-toxic phytoplankton species and mass occurrences of macro-algae can affect the amenity value of recreational waters due to reduced transparency, dis- coloured water and scum formation. Furthermore, bloom degradation can be accom- panied by unpleasant odours, resulting in aesthetic problems (see chapter 9). Several human diseases have been reported to be associated with many toxic species of dinoflagellates, diatoms, nanoflagellates and cyanobacteria that occur in the marine environment (CDC, 1997). The effects of these algae on humans are due to some of their constituents, principally algal toxins. Marine algal toxins become a problem primarily because they may concentrate in shellfish and fish that are subsequently eaten by humans (CDR, 1991; Lehane, 2000), causing syndromes known as para- lytic shellfish poisoning (PSP), diarrhetic shellfish poisoning (DSP), amnesic shellfish poisoning (ASP), neurotoxic shellfish poisoning (NSP) and ciguatera fish poisoning (CFP). -
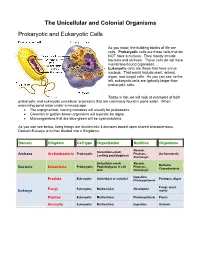
The Unicellular and Colonial Organisms Prokaryotic And
The Unicellular and Colonial Organisms Prokaryotic and Eukaryotic Cells As you know, the building blocks of life are cells. Prokaryotic cells are those cells that do NOT have a nucleus. They mostly include bacteria and archaea. These cells do not have membrane-bound organelles. Eukaryotic cells are those that have a true nucleus. That would include plant, animal, algae, and fungal cells. As you can see, to the left, eukaryotic cells are typically larger than prokaryotic cells. Today in lab, we will look at examples of both prokaryotic and eukaryotic unicellular organisms that are commonly found in pond water. When examining pond water under a microscope… The unpigmented, moving microbes will usually be protozoans. Greenish or golden-brown organisms will typically be algae. Microorganisms that are blue-green will be cyanobacteria. As you can see below, living things are divided into 3 domains based upon shared characteristics. Domain Eukarya is further divided into 4 Kingdoms. Domain Kingdom Cell type Organization Nutrition Organisms Absorb, Unicellular-small; Prokaryotic Photsyn., Archaeacteria Archaea Archaebacteria Lacking peptidoglycan Chemosyn. Unicellular-small; Absorb, Bacteria, Prokaryotic Peptidoglycan in cell Photsyn., Bacteria Eubacteria Cyanobacteria wall Chemosyn. Ingestion, Eukaryotic Unicellular or colonial Protozoa, Algae Protista Photosynthesis Fungi, yeast, Fungi Eukaryotic Multicellular Absorption Eukarya molds Plantae Eukaryotic Multicellular Photosynthesis Plants Animalia Eukaryotic Multicellular Ingestion Animals Prokaryotic Organisms – the archaea, non-photosynthetic bacteria, and cyanobacteria Archaea - Microorganisms that resemble bacteria, but are different from them in certain aspects. Archaea cell walls do not include the macromolecule peptidoglycan, which is always found in the cell walls of bacteria. Archaea usually live in extreme, often very hot or salty environments, such as hot mineral springs or deep-sea hydrothermal vents. -

About Cyanobacteria BACKGROUND Cyanobacteria Are Single-Celled Organisms That Live in Fresh, Brackish, and Marine Water
About Cyanobacteria BACKGROUND Cyanobacteria are single-celled organisms that live in fresh, brackish, and marine water. They use sunlight to make their own food. In warm, nutrient-rich environments, microscopic cyanobacteria can grow quickly, creating blooms that spread across the water’s surface and may become visible. Because of the color, texture, and location of these blooms, the common name for cyanobacteria is blue-green algae. However, cyanobacteria are related more closely to bacteria than to algae. Cyanobacteria are found worldwide, from Brazil to China, Australia to the United States. In warmer climates, these organisms can grow year-round. Scientists have called cyanobacteria the origin of plants, and have credited cyanobacteria with providing nitrogen fertilizer for rice and beans. But blooms of cyanobacteria are not always helpful. When these blooms become harmful to the environment, animals, and humans, scientists call them cyanobacterial harmful algal blooms (CyanoHABs). Freshwater CyanoHABs can use up the oxygen and block the sunlight that other organisms need to live. They also can produce powerful toxins that affect the brain and liver of animals and humans. Because of concerns about CyanoHABs, which can grow in drinking water and recreational water, the U.S. Environmental Protection Agency (EPA) has added cyanobacteria to its Drinking Water Contaminant Candidate List. This list identifies organisms and toxins that EPA considers to be priorities for investigation. ASSESSING THE IMPACT ON PUBLIC HEALTH Reports of poisonings associated with CyanoHABs date back to the late 1800s. Anecdotal evidence and data from laboratory animal research suggest that cyanobacterial toxins can cause a range of adverse human health effects, yet few studies have explored the links between CyanoHABs and human health. -

Rhythmicity of Coastal Marine Picoeukaryotes, Bacteria and Archaea Despite Irregular Environmental Perturbations
Rhythmicity of coastal marine picoeukaryotes, bacteria and archaea despite irregular environmental perturbations Stefan Lambert, Margot Tragin, Jean-Claude Lozano, Jean-François Ghiglione, Daniel Vaulot, François-Yves Bouget, Pierre Galand To cite this version: Stefan Lambert, Margot Tragin, Jean-Claude Lozano, Jean-François Ghiglione, Daniel Vaulot, et al.. Rhythmicity of coastal marine picoeukaryotes, bacteria and archaea despite irregular environmental perturbations. ISME Journal, Nature Publishing Group, 2019, 13 (2), pp.388-401. 10.1038/s41396- 018-0281-z. hal-02326251 HAL Id: hal-02326251 https://hal.archives-ouvertes.fr/hal-02326251 Submitted on 19 Nov 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Rhythmicity of coastal marine picoeukaryotes, bacteria and archaea despite irregular environmental perturbations Stefan Lambert, Margot Tragin, Jean-Claude Lozano, Jean-François Ghiglione, Daniel Vaulot, François-Yves Bouget, Pierre Galand To cite this version: Stefan Lambert, Margot Tragin, Jean-Claude Lozano, Jean-François Ghiglione, Daniel -

Phylogenomics Supports the Monophyly of the Cercozoa T ⁎ Nicholas A.T
Molecular Phylogenetics and Evolution 130 (2019) 416–423 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Phylogenomics supports the monophyly of the Cercozoa T ⁎ Nicholas A.T. Irwina, , Denis V. Tikhonenkova,b, Elisabeth Hehenbergera,1, Alexander P. Mylnikovb, Fabien Burkia,2, Patrick J. Keelinga a Department of Botany, University of British Columbia, Vancouver V6T 1Z4, British Columbia, Canada b Institute for Biology of Inland Waters, Russian Academy of Sciences, Borok 152742, Russia ARTICLE INFO ABSTRACT Keywords: The phylum Cercozoa consists of a diverse assemblage of amoeboid and flagellated protists that forms a major Cercozoa component of the supergroup, Rhizaria. However, despite its size and ubiquity, the phylogeny of the Cercozoa Rhizaria remains unclear as morphological variability between cercozoan species and ambiguity in molecular analyses, Phylogeny including phylogenomic approaches, have produced ambiguous results and raised doubts about the monophyly Phylogenomics of the group. Here we sought to resolve these ambiguities using a 161-gene phylogenetic dataset with data from Single-cell transcriptomics newly available genomes and deeply sequenced transcriptomes, including three new transcriptomes from Aurigamonas solis, Abollifer prolabens, and a novel species, Lapot gusevi n. gen. n. sp. Our phylogenomic analysis strongly supported a monophyletic Cercozoa, and approximately-unbiased tests rejected the paraphyletic topologies observed in previous studies. The transcriptome of L. gusevi represents the first transcriptomic data from the large and recently characterized Aquavolonidae-Treumulida-'Novel Clade 12′ group, and phyloge- nomics supported its position as sister to the cercozoan subphylum, Endomyxa. These results provide insights into the phylogeny of the Cercozoa and the Rhizaria as a whole.