Investigation of Wild Species Potential to Increase Genetic Diversity Useful for Apple Breeding
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Apple Breeding: a Study of Jonathan Crosses
J une, 1928 Research Bulletin No. 116 A.pple Breeding: A Study of Jonathan Crosses By H. L . LANTZ AGRICULTURAL EXPERIMENT STATION IOWA STATE COLLEGE OF AGRICULTURE AND MECHANIC ARTS C. F. CURTISS, Director POMOLOGY SECTION AMES, IOWA SUMMARY Two hundred seventy-three Jonathan seedlings produced by 11 crosses form the basis of the records in this bulletin. These studies show clearly that the progenies produced by the different crosses of Jonathan differ or vary in many important char acteristics. These variations are attributed to differences in the genetic constitution of the Eeveral varieties which were used in combination with Jonathan, and indicate the relative value of these varieties as parents when crossed with Jonathan. The range of variation in the seedlings of these progenies is gen erally quite wide both as to tree and fruit. Differences in the horti cultural characteristics such as occur in size, form, color, flavor, quality and season of the seedling fruits suggest that multiple fac tors are concerned, which produce the many variations observed. Jonathan appears to carry as partially dominant factors for medi um and below medium size of fruit, but it evidently carries factors also for large and for small size. Factors for roundish, conic, ob late and oblong fruit forms are present. Jonathan appears to be homozygous for red skin color but is heterozygous for pattern and carries factors for both fine and coarse grained flesh, for juiciness, for both acid and sweet flavor and for very good as well as for poor quality. The factors which control the season of the fruit are evi dently complex. -
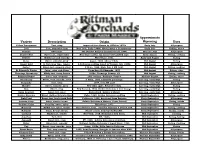
Variety Description Origin Approximate Ripening Uses
Approximate Variety Description Origin Ripening Uses Yellow Transparent Tart, crisp Imported from Russia by USDA in 1870s Early July All-purpose Lodi Tart, somewhat firm New York, Early 1900s. Montgomery x Transparent. Early July Baking, sauce Pristine Sweet-tart PRI (Purdue Rutgers Illinois) release, 1994. Mid-late July All-purpose Dandee Red Sweet-tart, semi-tender New Ohio variety. An improved PaulaRed type. Early August Eating, cooking Redfree Mildly tart and crunchy PRI release, 1981. Early-mid August Eating Sansa Sweet, crunchy, juicy Japan, 1988. Akane x Gala. Mid August Eating Ginger Gold G. Delicious type, tangier G Delicious seedling found in Virginia, late 1960s. Mid August All-purpose Zestar! Sweet-tart, crunchy, juicy U Minn, 1999. State Fair x MN 1691. Mid August Eating, cooking St Edmund's Pippin Juicy, crisp, rich flavor From Bury St Edmunds, 1870. Mid August Eating, cider Chenango Strawberry Mildly tart, berry flavors 1850s, Chenango County, NY Mid August Eating, cooking Summer Rambo Juicy, tart, aromatic 16th century, Rambure, France. Mid-late August Eating, sauce Honeycrisp Sweet, very crunchy, juicy U Minn, 1991. Unknown parentage. Late Aug.-early Sept. Eating Burgundy Tart, crisp 1974, from NY state Late Aug.-early Sept. All-purpose Blondee Sweet, crunchy, juicy New Ohio apple. Related to Gala. Late Aug.-early Sept. Eating Gala Sweet, crisp New Zealand, 1934. Golden Delicious x Cox Orange. Late Aug.-early Sept. Eating Swiss Gourmet Sweet-tart, juicy Switzerland. Golden x Idared. Late Aug.-early Sept. All-purpose Golden Supreme Sweet, Golden Delcious type Idaho, 1960. Golden Delicious seedling Early September Eating, cooking Pink Pearl Sweet-tart, bright pink flesh California, 1944, developed from Surprise Early September All-purpose Autumn Crisp Juicy, slow to brown Golden Delicious x Monroe. -

Apples: Organic Production Guide
A project of the National Center for Appropriate Technology 1-800-346-9140 • www.attra.ncat.org Apples: Organic Production Guide By Tammy Hinman This publication provides information on organic apple production from recent research and producer and Guy Ames, NCAT experience. Many aspects of apple production are the same whether the grower uses low-spray, organic, Agriculture Specialists or conventional management. Accordingly, this publication focuses on the aspects that differ from Published nonorganic practices—primarily pest and disease control, marketing, and economics. (Information on March 2011 organic weed control and fertility management in orchards is presented in a separate ATTRA publica- © NCAT tion, Tree Fruits: Organic Production Overview.) This publication introduces the major apple insect pests IP020 and diseases and the most effective organic management methods. It also includes farmer profiles of working orchards and a section dealing with economic and marketing considerations. There is an exten- sive list of resources for information and supplies and an appendix on disease-resistant apple varieties. Contents Introduction ......................1 Geographical Factors Affecting Disease and Pest Management ...........3 Insect and Mite Pests .....3 Insect IPM in Apples - Kaolin Clay ........6 Diseases ........................... 14 Mammal and Bird Pests .........................20 Thinning ..........................20 Weed and Orchard Floor Management ......20 Economics and Marketing ........................22 Conclusion -

An Old Rose: the Apple
This is a republication of an article which first appeared in the March/April 2002 issue of Garden Compass Magazine New apple varieties never quite Rosaceae, the rose family, is vast, complex and downright confusing at times. completely overshadow the old ones because, as with roses, a variety is new only until the next This complexity has no better exemplar than the prince of the rose family, Malus, better known as the variety comes along and takes its apple. The apple is older in cultivation than the rose. It presents all the extremes in color, size, fragrance place. and plant character of its rose cousin plus an important added benefit—flavor! One can find apples to suit nearly every taste and cultural demand. Without any special care, apples grow where no roses dare. Hardy varieties like the Pippins, Pearmains, Snow, Lady and Northern Spy have been grown successfully in many different climates across the U.S. With 8,000-plus varieties worldwide and with new ones introduced annually, apple collectors in most climates are like kids in a candy store. New, Favorite and Powerhouse Apples New introductions such as Honeycrisp, Cameo and Pink Lady are adapted to a wide range of climates and are beginning to be planted in large quantities. The rich flavors of old favorites like Spitzenburg and Golden Russet Each one is a unique eating experience that are always a pleasant surprise for satisfies a modern taste—crunchy firmness, plenty inexperienced tasters. of sweetness and tantalizing flavor. Old and antique apples distinguish These new varieties show promise in the themselves with unusual skin competition for the #1 spot in the world’s colors and lingering aftertastes produce sections and farmers’ markets. -
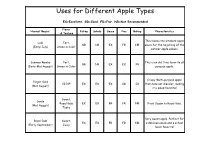
Uses for Different Apple Types
Uses for Different Apple Types EX=Excellent, GD=Good, FR=Fair, NR=Not Recommended Flavor Harvest Begins: Eating Salads Sauce Pies Baking Characteristics & Texture This makes the ultimate apple Lodi Tart, NR NR EX FR NR sauce for the beginning of the (Early July) Green in Color summer apple season. Summer Rambo Tart, This is an old-time favorite all- NR NR EX EX FR (Early-Mid August) Green in Color purpose apple. Crispy Multi-purpose apple Ginger Gold CRISP EX EX EX GD GD that does not discolor, making (Mid August) it a salad favorite! Sweet, Sansa Royal Gala EX EX FR FR NR First Cousin to Royal Gala. (Mid August) Taste Very sweet apple. Perfect for Royal Gala Sweet, EX EX FR FR NR a delicous snack and a school (Early September) Juicy lunch favorite! Extremely popular sweet Honey Crisp tasting apple. Our most crispy CRISP EX EX EX EX FR (Early September) and juiciest apple perfect for a sweet snack! MacIntosh Semi-Sweet/ General all purpose apple. EX GD EX EX FR (Mid September) Tart Nice sweet-tart apple. Exclusively sold at Milburn Orange Honey Sweet, EX EX EX EX FR Orchards. Some say equal to (Mid September) Crisp Honey Crisp! Crispy, tart flavor. This apple is available before Stayman Jonathan CRISP/ EX GD GD EX EX Winesap and a perfect (Mid September) Tart substitute. Multi-Purpose apple. Cortland Semi- Multi-Purpose apple. Next GD GD EX EX FR (Mid September) Tart best thing to MacIntosh. Sweet, An offspring of Fuji, same September Fuji Juicy, EX EX GD EX GD qualities but 4 weeks (Mid September) Not very earlier. -
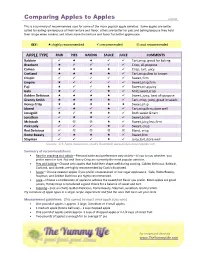
Comparing Apples to Apples 10/2010
Comparing Apples to Apples 10/2010 This is a summary of recommended uses for some of the more popular apple varieties. Some apples are better suited for eating raw because of their texture and flavor, others are better for pies and baking because they hold their shape when cooked, and others have the texture and flavor for better applesauce. KEY: =highly recommended =recommended =not recommended APPLE TYPE RAW PIES BAKING SAUCE JUICE COMMENTS Baldwin Tart,crisp, great for baking Braeburn Crisp, all-purpose Cameo Crisp, tart, juicy Cortland Tart,crisp;slow to brown Crispin Sweet, firm Empire Sweet,crisp,firm Fuji Sweet,crisp,juicy Gala Mild,sweet,crisp Golden Delicious Sweet, juicy, best all-purpose Granny Smith Tart, crisp, juicy, great in salads Honey Crisp Sweet,crisp Idared Tart,crisp,firm,store well Jonagold Both sweet & tart Jonathan Sweet,acidic McIntosh Sweet,juicy,less firm Pink Lady Sweet, crisp Red Delicious Bland, crisp Rome Beauty Sweet,firm Stayman Juicy,tart,store well Sources: U.S. Apple Association, Cook’s Illustrated, www.pickyourown/apples.com Summary of recommendations: • Raw for snacking and salads —Personal taste and preferences vary widely—it’s up to you whether you prefer sweet or tart. Fuji and Honey Crisp are currently the most popular varieties. • Pies and baking —Choose only apples that hold their shape well during cooking. Golden Delicious, Baldwin, Cortland, and Idareds are highly recommended by Cook’s Illustrated. • Sauce —Choose sweeter apples if you prefer unsweetened or low-sugar applesauce. Gala, Rome Beauty, Stayman, and Golden Delicious are highly recommended. • Juice —Choose a combination of apples to achieve the sweet/tart flavor you prefer. -

Factors' Influencing the Development of Soggy Break-Down in Apples ^
FACTORS' INFLUENCING THE DEVELOPMENT OF SOGGY BREAK-DOWN IN APPLES ^ By H. H. PLAGGE, research assistant professor, and T. J. MANEY, research professor and head, Subsection of Pomology, Iowa Agricultural Experiment Station INTRODUCTION The influence of storage temperature on soggy break-down ^ in certain apple varieties is generally recognized, but the relation of other etiologic factors to this disease is not so clearly understood. For example, it has been assumed that delayed storage is essential to the development of soggy break-down, and that late picking favors its occurrence. The effect of these and other causal agencies has not been demonstrated conclusively. Among other factors which have been considered as favorable or essential to the development of soggy break-down are inadequate ventilation of containers and storage rooms, a prolonged storage period, temperatures lower than 32° F., variations in fertilizer treatments, and climatic conditions. The effect of these and certain other factors on soggy break-down is con sidered in this paper. The importance of storage temperature in the control of soggy breakdown has been emphasized in earlier pubhcations {11, 16, 17)? This paper presents further evidence to show the relation of storage temperature to the disease in the varieties that were previously listed as susceptible and in other varieties for which little or no information is on record. The relative importance of other storage and prestorage factors as judged by the results of more than 11 years' work is indicated. REVIEW OF LITERATURE Investigators have emphasized the importance of immediate storage in the control of the soft scald type of soggy break-down in the Jonathan apple (Í, S, 6, 18y 19). -

INF03 Reduce Lists of Apple Varieites
ECE/TRADE/C/WP.7/GE.1/2009/INF.3 Specialized Section on Standardization of Fresh Fruit and Vegetables Fifty-fifth session Geneva, 4 - 8 May 2009 Items 4(a) of the provisional agenda REVISION OF UNECE STANDARDS Proposals on the list of apple varieties This note has been put together by the secretariat following the decision taken by the Specialized Section at its fifty-fourth session to collect information from countries on varieties that are important in international trade. Replies have been received from the following countries: Canada, Czech Republic, Finland, France, Germany, Italy, Netherlands, New Zealand, Poland, Slovakia, South Africa, Sweden, Switzerland and the USA. This note also includes the documents compiled for the same purpose and submitted to the fifty-second session of the Specialized Section. I. Documents submitted to the 52nd session of the Specialized Section A. UNECE Standard for Apples – List of Varieties At the last meeting the 51 st session of the Specialized Section GE.1 the delegation of the United Kingdom offered to coordinate efforts to simplify the list of apple varieties. The aim was to see what the result would be if we only include the most important varieties that are produced and traded. The list is designed to help distinguish apple varieties by colour groups, size and russeting it is not exhaustive, non-listed varieties can still be marketed. The idea should not be to list every variety grown in every country. The UK asked for views on what were considered to be the most important top thirty varieties. Eight countries sent their views, Italy, Spain, the Netherlands, USA, Slovakia, Germany Finland and the Czech Republic. -

Ashmead's Kernel Heirloom Apple
ORGANIC Ashmead’s Kernel Heirloom Apple • Homely to look at but truly memorable to eat; distinctive sweet sharp flavor with hint of pear drops. • Crisp, juicy flesh; highly aromatic. • Best eaten fresh, but can be cooked. • Highly valued for juicing and hard cider. DELIVERED FROM THE FARM BY ORGANIC Arkansas Black Heirloom Apple • Flesh is golden hued and juicy with a fine-grained, crisp texture. • Highly aromatic with sweet-tart flavor. • Excellent cooking apple; bake, saute or roast, puree into soups or sauces. • Juice is excellent for ciders and jams. • Pairs well with winter squash, pecans, DELIVERED FROM cranberries, vanilla, thyme, sage, THE FARM BY cinnamon and cardamom. ORGANIC King David Heirloom Apple • Crisp and juicy flesh is creamy yellow with a coarse texture. • Rich spicy flavor with nuances of wine compliments sweet and savory dishes. • Excellent cooking apple; bake, saute or roast, also great in stuffing. Perfect for cider, sauces, juice DELIVERED FROM • THE FARM BY and preserves. ORGANIC Jonathan Heirloom Apple • Thin skin with fine textured, creamy yellow flesh. • Juicy with a crisp bite; flavor is mildly sweet with a tart tang and hint of spice. • Great to eat raw or cooked in sweet or savory dishes; flesh breaks down slightly when cooked. • Pair with denser apples for pie or sauces. DELIVERED FROM • Perfect for use in juice or cider. THE FARM BY ORGANIC Sugar Pie Pumpkin • Choose item with smooth, orange skin and slight ridges. • Bright orange flesh is sweet; firm flesh cooks down to smooth consistency. • Specifically bred for baking. • High in the antioxidant beta-carotene, also provides protein, fiber, and calcium. -

Progress of Apple Breeding in Japan
Progress of Apple Breeding in Japan YOSHIO YOSHIDA Morioka Branch, Fruit Tree Research Station Introduction Breeding programs in the Aomori Apple Experiment Station In 1872, the Japanese Government imported 75 apple varieties from the United States of In 1928 the apple breeding program was America including Ralls Janet and Jonathan started and up to 1934, 5,267 hybrid seedlings which have been cultivated as two leading derived from 194 crosses using 50 parent commercial apple varieties in Japan up to the varieties were nursed. After long term selec present time. After that time Japan has tions Mutsu and other three varieties were imported about 900 apple varieties from released in 1948, Megumi, Orei and other foreign countries including McIntosh Red, eight varieties in 1949, Aori #1 in 1953, Toko Golden Delicious, Starking Delicious, Redgold, in 1963°, Aori #2 (Tsugaru) and Aori #3 Jonagold and so on">. Since their introduction in 1970'-,..>, Sekai-ichi in 197410>. Among them, in 1872, apple has become the second leading Mutsu, Megumi, Orei and Tsugaru (Aori #2) fruit crop, next citrus, with about 60,000 were registered with their variety name re hectares of area producing about one million spectively tons annually in Japan3 >. The second apple breeding program was There is considerable interest in the develop started in 1952 and up to 1960, 7,133 hybrid ment of new apple varieties that are better seedlings derived from 206 crosses were suited to the Japanese preference; sweet, tree nursed. Two strains of Starking Delicious ripened fruit and good keeping quality than X Tsugaru-#6 were released in 1969 and many of the "old" American varieties which Tsugaru x American Summer Pearmain-#9 constitute much of the present acreage. -

Fruits, Old and New and Northern Plant Novelties N
South Dakota State University Open PRAIRIE: Open Public Research Access Institutional Repository and Information Exchange South Dakota State University Agricultural Bulletins Experiment Station 3-1-1937 Fruits, Old and New and Northern Plant Novelties N. E. Hansen Follow this and additional works at: http://openprairie.sdstate.edu/agexperimentsta_bulletins Recommended Citation Hansen, N. E., "Fruits, Old and New and Northern Plant Novelties" (1937). Bulletins. Paper 309. http://openprairie.sdstate.edu/agexperimentsta_bulletins/309 This Bulletin is brought to you for free and open access by the South Dakota State University Agricultural Experiment Station at Open PRAIRIE: Open Public Research Access Institutional Repository and Information Exchange. It has been accepted for inclusion in Bulletins by an authorized administrator of Open PRAIRIE: Open Public Research Access Institutional Repository and Information Exchange. For more information, please contact [email protected]. Bulletin No. 309 ·March; 1937 Fruits, Old and New and Northern Plant Novelties By N. E. Hansen Department of Horticulture Agricultural Experiment Station South Dakota State College of Agriculture and Mechanic Arts Brookings, S. D. 2 BULLETIN 309 SOUTH DAKOTA EXPERIMENT STATION 1937 Hardy Fruit List for South Dakota By N. E. Hansen, Horticultur,ist This list of fruits recommended for. planting will vary because of local conditions. Planters in the extreme southern and southeastern edge of the state will be interested in the fruit lists of northern Nebraska and northern Iowa. For the eastern and northeastern counties, across the state, the North Dakota list should be studied. The Black Hills region is more sheltered and can grow many varieties not hardy on the open prairies further east. -

Americus Crab Americus Crab -Side Arkansas Black Arkansas Black -Side Ashmeads Kernel
Americus Crab Americus Crab -side Arkansas Black Arkansas Black -side Ashmeads Kernel Ashmeads Kernel -side Baldwin Baldwin -side Beacon Beacon -side Bella Vista Bella Vista -side Binet Blanc Binet Blanc -side Blenheim Orange Blenheim Orange -side Blushing Golden Blushing Golden -side BWO Early WI Red BWO Early WI Red -side BWO Sweet Thingy BWO Sweet Thingy -side Calville Blanc dHiver Calville Blanc dHiver -side Chenango Strawberry Chenango Strawberry -side Chestnut Crab Chestnut Crab -side Chisel Jersey (faux) -side Chisel Jersey (faux) Coat Jersey Coat Jersey -side Connell Red Connell Red -side Coos Bay Late Coos Bay Late -side Cornell Sour Cornell Sour -side Cornell Sweet Cornell Sweet -side Cortland Cortland -side Cox's Orange Pippin Cox's Orange Pippin -side Cricket Creek Yellow Crab Cricket Creek Yellow Crab - Crimson King Crimson King -side Crispin Crispin -side side Davey Davey -side Dolgo Crab Dolgo Crab -side Domaine Domaine -side DS-99 DS-99 -side DS 428 DS 428 -side Duchess of Oldenburg Duchess of Oldenburg -side Early Jon Early Jon -side Empire Empire -side English Golden Russet English Golden Russet -side Esopus Spitzenberg Esopus Spitzenberg -side Fireside Fireside - side Frequin Rouge Frequin Rouge -side Fuji Fuji -side Gala Gala -side Gallia Beauty Gallia Beauty -side Genesis II Genesis II -side Golden Delicious Golden Delicious -side Golden Nugget Golden Nugget -side Granny Smith Granny Smith -side Gravenstein Gravenstein -side Greendale Greendale -side Grimes Golden Grimes Golden -side Haralson Haralson -side Holdren's