Biomolecules
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Uvic Thesis Template
Corporatist Legislature: Authoritarianism, Representation and Local People‘s Congress in Zhejiang by Jing Qian LL.B., Zhejiang Gongshang University, 2007 A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of MASTER OF LAWS in the Faculty of Law Jing Qian, 2009 University of Victoria All rights reserved. This thesis may not be reproduced in whole or in part, by photocopy or other means, without the permission of the author. ii Supervisory Committee Corporatist Legislature: Authoritarianism, Representation and Local People‘s Congress in Zhejiang by Jing Qian LL.B., Zhejiang Gongshang University, 2007 Supervisory Committee Dr. Andrew James Harding, Faculty of Law, University of Victoria Co-Supervisor Dr. Guoguang Wu, Department of Political Science, University of Victoria Co-Supervisor iii Abstract Supervisory Committee Dr. Andrew James Harding, Faculty of Law, University of Victoria Co-Supervisor Dr. Guoguang Wu, Department of Political Science, University of Victoria Co-Supervisor In this thesis, the author analyzes the role of Local People‘s Congresses (LPCs) in China in shaping state-society relations in a decentralized authoritarian regime. Classical theories of corporatism are applied in order to examine the political functions of the LPC, a local representative and legislative mechanism. The author further proposes expanding the application of corporatist theory to encompass elected representative assemblies. In his analysis, the author explores how the state penetrates into and controls the LPC, and how, at the same time, the local legislature unequally incorporates various social groups into public affairs. He compares and contrasts biased strategies adopted by the state via the LPCs concerning different social sectors, under a dichotomy of inclusionary and exclusionary corporatism, based on which he further suggests a tentative typology of liberal/corporatist/communist legislature to enrich theories of comparative legislative studies. -
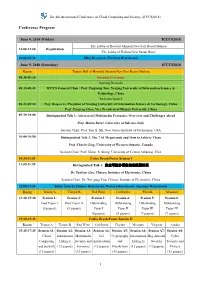
Conference Program
The 4th International Conference on Cloud Computing and Security (ICCCS2018) Conference Program June 8, 2018 (Friday) ICCCS2018 The Lobby of Howard Johnson New Port Resort Haikou 14:00-21:00 Registration The Lobby of Haikou New Yantai Hotel 18:00-21:30 BBQ Reception (Western Restaurant) June 9, 2018 (Saturday) ICCCS2018 Room Tianjin Hall of Howard Johnson New Port Resort Haikou 08:30-09:10 Opening Ceremony Opening Remarks 08:35-08:45 ICCCS General Chair: Prof. Xingming Sun, Nanjing University of Information Science & Technology, China Welcome Speech 08:45-09:10 Prof. Beiqun Li, President of Nanjing University of Information Science & Technology, China Prof. Xianfeng Chen, Vice President of Hainan University, China 09:10-10:00 Distinguished Talk 1: Adversarial Multimedia Forensics: Overview and Challenges Ahead Prof. Mauro Barni, University of Salerno, Italy Session Chair: Prof. Yun Q. Shi, New Jersey Institute of Technolgoy, USA 10:00-10:50 Distinguished Talk 2: The 7 AI Megatrends and How to Achieve Them Prof. Charles Ling, University of Western Ontario, Canada Session Chair: Prof. Victor. S. Sheng, University of Central Arkansas, USA 10:50-11:05 Coffee Break/Poster Session I 11:05-11:55 Distinguished Talk 3: 安全可控多模态信息隐藏体系 Dr. Yunbiao Guo, Chinese Institute of Electronics, China Session Chair: Dr. Xin’gang You, Chinese Institute of Electronics, China 12:00-13:30 Buffet Lunch (Chinese Restaurant, Western Restaurant, Japanese Restaurant) Room Tianjin A Tianjin B Red Wine California Florida Missouri 13:30-15:30 Session 1: Session 2: Session -

"Du Shiniang Sinks Her Jewel Box in Anger": a Story to Defend Folk
"Du Shiniang Sinks Her Jewel Box in Anger": A Story to Defend Folk Literature Presented to the Faculty of the Department of East Asian Languages and Cultures Bryn Mawr College In partial fulfillment of the requirement for the degree of Bachelors of Arts By Binglei Yan Advisor: Professor Shiamin Kwa Bryn Mawr, Pennsylvania December 2015 Abstract This thesis takes a look at one of the short stories in Feng Menglong's Sanyan collection, "Du Shiniang Sinks Her Jewel Box in Anger." Written during the late Ming dynasty, the story has been typically analyzed by present-day scholars as a political allegory or as a lesson to teach qing, a term which translated alternately as "passions," "love," or "romantic sentiments" in English. Based on the background that the archaic elite literature was advocated through the Ming literary movement called "the restoration of the past" and Feng Menglong, as a follower of key anti-archaists like Wang Yanming, Li Zhi, and Yuan Hongdao, emphasized authentic feelings and spontaneity in literature, this thesis argues that in "Du Shiniang Sinks Her Jewel Box in Anger," Feng Menglong metaphorically defended folk literature by defending Du Shiniang. Through examining the ways in which Feng Menglong praised the courtesan Du Shiniang's spontaneous and sincere nature that embodied in her xia (chivalry) and qing characteristics in the story, it becomes clear that Feng Menglong advocated folk literature as what should be extolled in the late Ming. The thesis concludes by recommending that this Feng Menglong's story is possibly a forerunner of a growing genre in the Qing dynasty which makes it worth for further researches. -

Transcriptome Sequencing Reveals the Differentially Expressed Lncrnas and Mrnas Involved in Cryoinjuries in Frozen-Thawed Giant Panda (Ailuropoda Melanoleuca) Sperm
International Journal of Molecular Sciences Article Transcriptome Sequencing Reveals the Differentially Expressed lncRNAs and mRNAs Involved in Cryoinjuries in Frozen-Thawed Giant Panda (Ailuropoda melanoleuca) Sperm Ming-Xia Ran 1,†, Yuan Li 1,†, Yan Zhang 1, Kai Liang 1, Ying-Nan Ren 1, Ming Zhang 1, Guang-Bin Zhou 1, Ying-Min Zhou 2, Kai Wu 2, Cheng-Dong Wang 2, Yan Huang 2, Bo Luo 2, Izhar Hyder Qazi 1,3 , He-Min Zhang 2 and Chang-Jun Zeng 1,* 1 College of Animal Sciences and Technology, Sichuan Agricultural University, Chengdu 611130, Sichuan, China; [email protected] (M.-X.R.); [email protected] (Y.L.); [email protected] (Y.Z.); [email protected] (K.L.); [email protected] (Y.-N.R.); [email protected] (M.Z.); [email protected] (G.-B.Z.); [email protected] (I.H.Q.) 2 China Conservation and Research Center for the Giant Panda, Wolong 473000, China; [email protected] (Y.-M.Z.); [email protected] (K.W.); [email protected] (C.-D.W.); [email protected] (Y.H.); [email protected] (B.L.); [email protected] (H.-M.Z.) 3 Department of Veterinary Anatomy & Histology, Faculty of Bio-Sciences, Shaheed Benazir Bhutto University of Veterinary and Animal Sciences, Sakrand 67210, Pakistan * Correspondence: [email protected]; Tel./Fax: +86-28-8629-1010 † These authors contribute equally to this work. Received: 5 September 2018; Accepted: 5 October 2018; Published: 8 October 2018 Abstract: Sperm cryopreservation and artificial insemination are important methods for giant panda breeding and preservation of extant genetic diversity. Lower conception rates limit the use of artificial insemination with frozen-thawed giant panda sperm, due to the lack of understanding of the cryodamaging or cryoinjuring mechanisms in cryopreservation. -

The Historical Foundations of Religious Restrictions in Contemporary China
religions Article The Historical Foundations of Religious Restrictions in Contemporary China Yu Tao School of Social Sciences, The University of Western Australia, M257, 35 Stirling Highway, Crawley, WA 6009, Australia; [email protected] Received: 12 October 2017; Accepted: 29 November 2017; Published: 1 December 2017 Abstract: The ruling Chinese Communist Party (CCP) abolished its total ban on religious activities in 1982. However, the distrust that the CCP feels for religions remains obvious today, and the religious restrictions in contemporary China remain tight. Conventional wisdom tells us that the official atheist ideology of Marxism-Leninism is the main reason behind the CCP’s distrust for, and restriction of, religion. However, taking a historical institutionalist perspective, this paper argues that the religious restrictions in contemporary China are in fact rooted in the fierce political struggles of the country’s two major revolutions in the first half of the twentieth century. Without the support of religious groups, the Nationalist Republicans would have found it difficult to survive and succeed in overthrowing the Qing Dynasty during the Chinese Republican Revolution in the first decade of the twentieth century. Likewise, without cooperating with a wide range of religious groups, the CCP would have struggled to defeat the Nationalist regime and the Japanese invaders in the Chinese Communist Revolution between 1920s and 1940s. Thanks to the collaborations and struggles with various religious groups during the two revolutions which lead to its eventual ascent to power, the CCP thoroughly understands the organisational strength and mobilising capability embedded within religious groups. The tight restrictions on religious affairs in contemporary China is therefore likely to stem from the CCP’s worry that prospective competitors could mobilise religious groups to challenge its rule through launching, supporting, or sponsoring collective actions. -

JRC 2010 Final Program
2010 Joint Rail Conference High-Speed and Intercity Passenger Rail Final Program April 27-29, 2010 Urbana, IL ILLINOIS r RAILROAD ENGINEERING PROGRAM Supporters 2 Chair’s Welcome Message Welcome to the 2010 Joint Rail Conference (JRC) hosted by the Railroad Engineering Program at the University of Illinois at Urbana- Champaign. The 2010 Joint Rail Conference is a cooperative eff ort of the rail groups of the American Society of Civil Engineers (ASCE), American Society of Mechanical Engineers (ASME), Institute of Electrical and Electronics Engineers (IEEE), American Railway Engineer- ing and Maintenance of Way Association (AREMA) and the Transportation Research Board (TRB) of the National Academies, repre- senting the leading rail transportation engineering educational and academic research organizations in North America. The expertise and perspective off ered by these organizations has led to a conference that has both breadth and depth in a wide range of current rail transportation and engineering topics. In light of the new North American focus on developing High-speed and Intercity Passenger Rail, the organizers selected this as the 2010 conference theme. A worldwide call for papers and presentations on both passenger and freight rail subjects was issued, requesting abstracts on all aspects of rail transport. The response was overwhelming; over 270 abstracts were received from six continents and 23 diff erent countries on a wide variety of rail engineering, transportation, management, planning, safety and operations topics. The conference program will include approximately 200 spoken presentations, a special poster presentation session, invited speakers and plenary sessions. Session topics will cover a wide range of passenger and freight railroad civil, mechanical, electrical and systems engineering topics, as well as rail safety, planning, design, fi nancing, operations and management. -

Die Xia-Dynastie in Sichuan (1362-1371)
Die Xia-Dynastie in Sichuan (1362-1371) Wissenschaftliche Hausarbeit zur Erlangung des akademischen Grades eines Magister Artium der Universität Hamburg vorgelegt von Max Jakob Fölster aus Dannenberg/Elbe Hamburg 2009 Inhaltsverzeichnis 1. Einleitung....................................................................................................................................1-6 1.1. Quellen ...................................................................................................................................1 1.2. Forschungsstand .....................................................................................................................4 1.3. Aufbau der Arbeit....................................................................................................................6 2. Chronologische Darstellung......................................................................................................7-60 2.1. Die sozialen Bedingungen am Ende der Yuan-Dynastie........................................................7 2.2. Die Aufstände der Roten Turbane am Ende der Yuan-Dynastie.............................................8 Die Nördlichen Roten Turbane.................................................................................................8 Die Südlichen Roten Turbane.................................................................................................10 2.3. Ming Yuzhen.........................................................................................................................14 -

Migration in the People's Republic of China
A Service of Leibniz-Informationszentrum econstor Wirtschaft Leibniz Information Centre Make Your Publications Visible. zbw for Economics Lu, Ming; Xia, Yiran Working Paper Migration in the People's Republic of China ADBI Working Paper, No. 593 Provided in Cooperation with: Asian Development Bank Institute (ADBI), Tokyo Suggested Citation: Lu, Ming; Xia, Yiran (2016) : Migration in the People's Republic of China, ADBI Working Paper, No. 593, Asian Development Bank Institute (ADBI), Tokyo This Version is available at: http://hdl.handle.net/10419/161469 Standard-Nutzungsbedingungen: Terms of use: Die Dokumente auf EconStor dürfen zu eigenen wissenschaftlichen Documents in EconStor may be saved and copied for your Zwecken und zum Privatgebrauch gespeichert und kopiert werden. personal and scholarly purposes. Sie dürfen die Dokumente nicht für öffentliche oder kommerzielle You are not to copy documents for public or commercial Zwecke vervielfältigen, öffentlich ausstellen, öffentlich zugänglich purposes, to exhibit the documents publicly, to make them machen, vertreiben oder anderweitig nutzen. publicly available on the internet, or to distribute or otherwise use the documents in public. Sofern die Verfasser die Dokumente unter Open-Content-Lizenzen (insbesondere CC-Lizenzen) zur Verfügung gestellt haben sollten, If the documents have been made available under an Open gelten abweichend von diesen Nutzungsbedingungen die in der dort Content Licence (especially Creative Commons Licences), you genannten Lizenz gewährten Nutzungsrechte. may exercise further usage rights as specified in the indicated licence. https://creativecommons.org/licenses/by-nc-nd/3.0/igo/ www.econstor.eu ADBI Working Paper Series Migration in the People’s Republic of China Ming Lu and Yiran Xia No. -

Manipulating Aeroponically Grown Potatoes with Gibberellins and Calcium Nitrate
American Journal of Potato Research https://doi.org/10.1007/s12230-018-9635-3 Manipulating Aeroponically Grown Potatoes with Gibberellins and Calcium Nitrate Cui-Cun Wang1,2 & Xi-Yao Wang3 & Ke-Xiu Wang1 & Jian-Jun Hu1 & Ming-Xia Tang1 & Wei He1 & Peter Vander Zaag4,5 # The Potato Association of America 2018 Abstract Maximizing tuber number and yield in an aeroponics production system is imperative to make it cost effective and to make the best use of all facilities and inputs. Manipulating the plants hormonal makeup is a strategy. GA3(GA), Calcium Nitrate(CaN) and their combination were foliarly applied several times to potato plants of cvs. Favorita and Mira during both the autumn and spring growing seasons in a semi tropical setting in Sichuan. A contrasting treatment of an anti-gibberellin(Anti) was also studied in both seasons. Significant differences in plants height, stem diameter, chlorophyll content, leaf area index, the number of stolons, the number of stolon branches and tuber production were observed among different treatments and cultivars. For the cv. Favorita, CaN + GA and GA increased tuber weight per plant by 63 and 49% in autumn and 53 and 73% in spring respectively as compared to the control(CK); tuber number per plant under CaN + GA and GA treatments increased by 90 and 85% in autumn and 35 and 52% in spring respectively. For cv. Mira, the trend was similar but not as dramatic. Tuber numbers over 1400 per square meter were harvested with cv. Mira during the spring season with the GA and CaN + GA treatments. Resumen La maximización del número de tubérculos y el rendimiento en un sistema de producción aeropónico es imperativo para hacerlo redituable y para hacer el mejor uso de todas las facilidades e insumos. -

Japanese Ceramics Latest Acquisitions
HANSHAN TANG BOOKS • L IST 161 NEW PUBLICATIONS MING CERAMICS JAPANESE CERAMICS LATEST ACQUISITIONS H ANSHAN TANG B OOKS LTD Unit 3, Ashburton Centre 276 Cortis Road London SW 15 3 AY UK Tel (020) 8788 4464 Fax (020) 8780 1565 Int’l (+44 20) [email protected] www.hanshan.com CONTENTS N EW & R ECENT P UBLICATIONS / 3 M ING C ERAMICS / 6 J APANESE C ERAMICS / 12 F ROM O UR S TOCK / 25 S UBJECT I NDEX / 60 T ERMS The books advertised in this list are antiquarian, second-hand or new publications. All books listed are in mint or good condition unless otherwise stated. If an out-of-print book listed here has already been sold, we will keep a record of your order and, when we acquire another copy, we will offer it to you. If a book is in print but not immediately available, it will be sent when new stock arrives. We will inform you when a book is not available. Prices take account of condition; they are net and exclude postage. Please note that we have occasional problems with publishers increasing the prices of books on the actual date of publication or supply. For secondhand items, we set the prices in this list. However, for new books we must reluctantly reserve the right to alter our advertised prices in line with any suppliers’ increases. P OSTAL C HARGES & D ISPATCH United Kingdom: For books weighing over 700 grams, minimum postage within the UK is GB £10.00. If books are lighter and we are able to charge less for delivery, we will do so. -

Download Article
Advances in Social Science, Education and Humanities Research, volume 310 3rd International Conference on Culture, Education and Economic Development of Modern Society (ICCESE 2019) The Origin and Development of Fuzhou Sea God Belief Liqin Fan Scientific Research Department Minjiang University Fuzhou, China 350108 Abstract—The belief of Fuzhou sea god is an important part culture formed by them in the navigation is deeply influenced of Fuzhou folk belief and one of the most oceanic features in by the marine culture. Among them, the folk belief of the sea Fujian capital culture. Its content is rich and varied, including god is the most prominent. Mazu Belief, Linshui Madame Belief, Shangshugong Chen Wenlong Belief, Yungong Belief and Yanyu God Belief. The Sea God belief is a phenomenon of god belief produced by belief in Fuzhou Sea God began in Song Dynasty with the people in the ocean activities. Fuzhou Sea God belief is an development of social economy, frequent maritime activities and important part of Fuzhou folk beliefs and it is also one of the the gradual development of overseas trade. With the promotion parts with the most maritime features. After Tang and Song of Fuzhou Port's status, the development of Fuzhou's navigation, Dynasties, with the increasing frequency of sea-related the further strengthening of overseas trade and the promotion of activities, Fuzhou Sea God belief gradually emerged. After the Zheng He's maritime activities, such as the envoys of Chengfeng Ming and Qing Dynasties, with the promotion of the status of Ryukyu, the belief in Fuzhou Sea God flourished in Ming and Fuzhou Port, the development of Fuzhou's navigation industry, Qing Dynasties. -

The Human Rights Crisis Tibet: Europe Must
The Human Rights Crisis in Tibet: Europe Must Act! BRIEFING NOTE The European Solidarity Rally for Tibet - 2012 May 2012 By Society for Threatened Peoples International Under Chinese domination Tibet1continues to remain in a state of constant fear, intimidation and persecution with the situation is deteriorating, day by day. Since the 2008 Uprising on the Tibetan Plateau, the human rights crisis faced the six million Tibetan people has been ignited by a series of intensified repressive policies and campaigns like the “people’s war” launched by the Chinese authorities, making life unbearable for Tibetans. Nomads are forcibly evicted from their ancestral lands, the precious natural resources of the Tibetan Plateau has led to destructive exploitation and exploration, huge numbers of security personnel have been deployed into the monasteries where forced indoctrination “patriotic re-education” sessions are conducted, censorship, propaganda and curtailment of the freedom of expression are vigorously enforced and Tibetans demanding freedom and the return of H. H. the Dalai Lama to Tibet are persecuted, detained under enforced disappearances and imprisoned with impunity facing the threat of torture and custodial deaths. More than thirty Tibetans, mostly monks and nuns have chosen self-immolation as the most drastic form of protest, to express their frustration about the Chinese oppression in Tibet. Even gatherings to pray for the dead ones are brutally suppressed by the Chinese authorities. On the other hand, the Sino-Tibetan dialogue has not resumed since February, 2010 while the Chinese authorities have failed to heed to the appeals that independent international experts be allowed to investigate the situation in Tibet.