Progress in EGFR-Mutant NSCLC: Where Are We Going? Transcript from a Touchexpert OPINIONS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Targeting the Epidermal Growth Factor Receptor in EGFR-Mutated Lung Cancer: Current and Emerging Therapies
cancers Review Targeting the Epidermal Growth Factor Receptor in EGFR-Mutated Lung Cancer: Current and Emerging Therapies Karam Khaddour 1,*, Sushma Jonna 1, Alexander Deneka 2 , Jyoti D. Patel 3, Mohamed E. Abazeed 4, Erica Golemis 2 , Hossein Borghaei 5 and Yanis Boumber 3,6,* 1 Division of Hematology and Oncology, University of Illinois at Chicago, Chicago, IL 60612, USA; [email protected] 2 Fox Chase Cancer Center, Program in Molecular Therapeutics, Philadelphia, PA 19111, USA; [email protected] (A.D.); [email protected] (E.G.) 3 Robert H. Lurie Comprehensive Cancer Center, Division of Hematology/Oncology, Feinberg School of Medicine, Northwestern University, Chicago, IL 60611, USA; [email protected] 4 Robert H. Lurie Comprehensive Cancer Center, Department of Radiation Oncology, Feinberg School of Medicine, Northwestern University, Chicago, IL 60611, USA; [email protected] 5 Fox Chase Cancer Center, Department of Hematology and Oncology, Philadelphia, PA 19111, USA; [email protected] 6 Institute of Fundamental Medicine and Biology, Kazan Federal University, 420008 Kazan, Russia * Correspondence: [email protected] (K.K.); [email protected] (Y.B.) Simple Summary: Epidermal growth factor receptor (EGFR) mutations occur in a significant number Citation: Khaddour, K.; Jonna, S.; of lung cancer patients. Treatment outcomes in this subset of patients has greatly improved over the Deneka, A.; Patel, J.D.; Abazeed, M.E.; last decade after the introduction of EGFR tyrosine kinase inhibitors (TKIs), which demonstrated high Golemis, E.; Borghaei, H.; Boumber, Y. efficacy and improved survival in randomized clinical trials. Although EGFR TKIs became the stan- Targeting the Epidermal Growth dard of care in patients with EGFR-mutated lung cancer, resistance almost inevitably develops. -

Interim Report for the First Quarter of 2020
Genmab Announces Financial Results for the First Quarter of 2020 May 6, 2020; Copenhagen, Denmark; Interim Report for the First Quarter Ended March 31, 2020 Highlights DARZALEX® (daratumumab) net sales increased approximately 49% compared to the first quarter of 2019 to USD 937 million, resulting in royalty income of DKK 775 million DARZALEX approved in Europe in combination with bortezomib, thalidomide and dexamethasone for the treatment of adult patients with newly diagnosed multiple myeloma who are eligible for autologous stem cell transplant U.S. FDA approved TEPEZZA™ (teprotumumab-trbw), developed and commercialized by Horizon Therapeutics, for thyroid eye disease U.S. FDA accepted, with priority review, Novartis’ supplemental Biologics License Application for subcutaneous ofatumumab in relapsing multiple sclerosis Anthony Pagano appointed Chief Financial Officer Anthony Mancini appointed Chief Operating Officer “Despite the unprecedented challenges posed by the coronavirus (COVID-19) pandemic, we will continue to invest in our innovative proprietary products, technologies and capabilities and use our world-class expertise in antibody drug development to create truly differentiated products with the potential to help cancer patients. While Genmab is closely monitoring the developments in the rapidly evolving landscape, we are extremely fortunate to have a solid financial foundation and a fabulous and committed team to carry us through these uncertain times,” said Jan van de Winkel, Ph.D., Chief Executive Officer of Genmab. Financial Performance First Quarter of 2020 Revenue was DKK 892 million in the first quarter of 2020 compared to DKK 591 million in the first quarter of 2019. The increase of DKK 301 million, or 51%, was mainly driven by higher DARZALEX royalties. -

Erbitux® (Cetuximab)
Erbitux® (cetuximab) (Intravenous) -E- Document Number: MODA-0494 Last Review Date: 06/01/2021 Date of Origin: 09/03/2019 Dates Reviewed: 09/2019, 01/2020, 04/2020, 07/2020, 10/2020, 01/2021, 04/2021, 06/2021 I. Length of Authorization 1 Coverage will be provided for six months and may be renewed unless otherwise specified. • SCCHN in combination with radiation therapy: Coverage will be provided for the duration of radiation therapy (6-7 weeks). II. Dosing Limits A. Quantity Limit (max daily dose) [NDC Unit]: Weekly Every two weeks Erbitux 100 mg/50 mL solution for injection 1 vial every 7 days 1 vial every 14 days 3 vials every 7 days Erbitux 200 mg/100 mL solution for injection 6 vials every 14 days (5 vials for first dose only) B. Max Units (per dose and over time) [HCPCS Unit]: Weekly Every two weeks − Load: 100 billable units x 1 dose 120 billable units every 14 days − Maintenance Dose: 60 billable units every 7 days III. Initial Approval Criteria 1 Coverage is provided in the following conditions: • Patient is at least 18 years of age; AND Colorectal Cancer (CRC) † ‡ 1,2,12,13,17,19,2e,5e-8e,10e-12e,15e • Patient is both KRAS and NRAS mutation negative (wild-type) as determined by FDA- approved or CLIA-compliant test*; AND • Will not be used as part of an adjuvant treatment regimen; AND • Patient has not been previously treated with cetuximab or panitumumab; AND • Will not be used in combination with an anti-VEGF agent (e.g., bevacizumab, ramucirumab); AND Moda Health Plan, Inc. -

Primary and Acquired Resistance to Immunotherapy in Lung Cancer: Unveiling the Mechanisms Underlying of Immune Checkpoint Blockade Therapy
cancers Review Primary and Acquired Resistance to Immunotherapy in Lung Cancer: Unveiling the Mechanisms Underlying of Immune Checkpoint Blockade Therapy Laura Boyero 1 , Amparo Sánchez-Gastaldo 2, Miriam Alonso 2, 1 1,2,3, , 1,2, , José Francisco Noguera-Uclés , Sonia Molina-Pinelo * y and Reyes Bernabé-Caro * y 1 Institute of Biomedicine of Seville (IBiS) (HUVR, CSIC, Universidad de Sevilla), 41013 Seville, Spain; [email protected] (L.B.); [email protected] (J.F.N.-U.) 2 Medical Oncology Department, Hospital Universitario Virgen del Rocio, 41013 Seville, Spain; [email protected] (A.S.-G.); [email protected] (M.A.) 3 Centro de Investigación Biomédica en Red de Cáncer (CIBERONC), 28029 Madrid, Spain * Correspondence: [email protected] (S.M.-P.); [email protected] (R.B.-C.) These authors contributed equally to this work. y Received: 16 November 2020; Accepted: 9 December 2020; Published: 11 December 2020 Simple Summary: Immuno-oncology has redefined the treatment of lung cancer, with the ultimate goal being the reactivation of the anti-tumor immune response. This has led to the development of several therapeutic strategies focused in this direction. However, a high percentage of lung cancer patients do not respond to these therapies or their responses are transient. Here, we summarized the impact of immunotherapy on lung cancer patients in the latest clinical trials conducted on this disease. As well as the mechanisms of primary and acquired resistance to immunotherapy in this disease. Abstract: After several decades without maintained responses or long-term survival of patients with lung cancer, novel therapies have emerged as a hopeful milestone in this research field. -

The Angiopoietin-2 and TIE Pathway As a Therapeutic Target for Enhancing Antiangiogenic Therapy and Immunotherapy in Patients with Advanced Cancer
International Journal of Molecular Sciences Review The Angiopoietin-2 and TIE Pathway as a Therapeutic Target for Enhancing Antiangiogenic Therapy and Immunotherapy in Patients with Advanced Cancer Alessandra Leong and Minah Kim * Department of Pathology and Cell Biology, Columbia University Irving Medical Center, New York, NY 10032, USA; afl[email protected] * Correspondence: [email protected] Received: 26 September 2020; Accepted: 13 November 2020; Published: 18 November 2020 Abstract: Despite significant advances made in cancer treatment, the development of therapeutic resistance to anticancer drugs represents a major clinical problem that limits treatment efficacy for cancer patients. Herein, we focus on the response and resistance to current antiangiogenic drugs and immunotherapies and describe potential strategies for improved treatment outcomes. Antiangiogenic treatments that mainly target vascular endothelial growth factor (VEGF) signaling have shown efficacy in many types of cancer. However, drug resistance, characterized by disease recurrence, has limited therapeutic success and thus increased our urgency to better understand the mechanism of resistance to inhibitors of VEGF signaling. Moreover, cancer immunotherapies including immune checkpoint inhibitors (ICIs), which stimulate antitumor immunity, have also demonstrated a remarkable clinical benefit in the treatment of many aggressive malignancies. Nevertheless, the emergence of resistance to immunotherapies associated with an immunosuppressive tumor microenvironment has restricted therapeutic response, necessitating the development of better therapeutic strategies to increase treatment efficacy in patients. Angiopoietin-2 (ANG2), which binds to the receptor tyrosine kinase TIE2 in endothelial cells, is a cooperative driver of angiogenesis and vascular destabilization along with VEGF. It has been suggested in multiple preclinical studies that ANG2-mediated vascular changes contribute to the development and persistence of resistance to anti-VEGF therapy. -

Cyramza® (Ramucirumab)
Cyramza® (ramucirumab) (Intravenous) -E- Document Number: MODA-0405 Last Review Date: 07/01/2021 Date of Origin: 09/03/2019 Dates Reviewed: 09/2019, 10/2019, 01/2020, 04/2020, 07/2020, 10/2020, 01/2021, 04/2021, 07/2021 I. Length of Authorization Coverage will be provided for 6 months and may be renewed. II. Dosing Limits A. Quantity Limit (max daily dose) [NDC Unit]: • Cyramza 100 mg/10 mL: 4 vials per 14 days • Cyramza 500 mg/50 mL: 2 vials per 14 days B. Max Units (per dose and over time) [HCPCS Unit]: Gastric, Gastroesophageal, HCC, and Colorectal Cancer: • 180 billable units every 14 days NSCLC: • 240 billable units every 14 days III. Initial Approval Criteria 1 Coverage is provided in the following conditions: • Patient is at least 18 years of age; AND Universal Criteria 1 • Patient does not have uncontrolled severe hypertension; AND • Patient must not have had a surgical procedure within the preceding 28 days or have a surgical wound that has not fully healed; AND Gastric, Esophageal, and Gastro-esophageal Junction Adenocarcinoma † Ф 1-3,5-7,14,17,2e,5e • Used as subsequent therapy after fluoropyrimidine- or platinum-containing chemotherapy; AND • Used as a single agent OR in combination with paclitaxel; AND o Used for one of the following: Moda Health Plan, Inc. Medical Necessity Criteria Page 1/27 Proprietary & Confidential © 2021 Magellan Health, Inc. – Patient has unresectable locally advanced, recurrent, or metastatic disease; OR – Used as palliative therapy for locoregional disease in patients who are not surgical candidates -
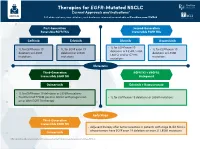
Therapies for EGFR-Mutated NSCLC Current Approvals and Indications1 Full Abbreviations, Accreditation, and Disclosure Information Available at Peerview.Com/CWE40
Therapies for EGFR-Mutated NSCLC 1 Current Approvals and Indications Full abbreviations, accreditation, and disclosure information available at PeerView.com/CWE40 First-Generation Second-Generation Reversible EGFR TKIs Irreversible EGFR TKIs Getinib Erlotinib Afatinib Dacomitinib • 1L for EGFR exon 19 • 1L for EGFR exon 19 • 1L for EGFR exon 19 • 1L for EGFR exon 19 deletions or L858R, S768I, deletions or L858R deletions or L858R deletions or L858R L861Q, and/or G719X mutations mutations mutations mutations Metastatic Third-Generation EGFR TKI + VEGFR2 Irreversible EGFR TKI Antagonist Osimertinib Erlotinib + Ramucirumab • 1L for EGFR exon 19 deletions or L858R mutations • Treatment of T790M-positive NSCLC with progression • 1L for EGFR exon 19 deletions or L858R mutations on or after EGFR TKI therapy Early Stage Third-Generation Irreversible EGFR TKI • Adjuvant therapy after tumor resection in patients with stage IB-IIIA NSCLC whose tumors have EGFR exon 19 deletions or exon 21 L858R mutations Osimertinib 1. https://www.fda.gov/drugs/resources-information-approved-drugs/hematologyoncology-cancer-approvals-safety-notifications. Molecular Testing Guidelines for NSCLC Latest Updates, Best Practices, and Patient-Reported Insights1 Full abbreviations, accreditation, and disclosure information available at PeerView.com/CWE40 Why Test Lung Cancer Patients for Genomic Alterations? • Genomic alterations are common in nonsquamous NSCLC (approximately 50%) • Targeted therapies produce better treatment outcomes (eg, higher response rates, improved -

The Two Tontti Tudiul Lui Hi Ha Unit
THETWO TONTTI USTUDIUL 20170267753A1 LUI HI HA UNIT ( 19) United States (12 ) Patent Application Publication (10 ) Pub. No. : US 2017 /0267753 A1 Ehrenpreis (43 ) Pub . Date : Sep . 21 , 2017 ( 54 ) COMBINATION THERAPY FOR (52 ) U .S . CI. CO - ADMINISTRATION OF MONOCLONAL CPC .. .. CO7K 16 / 241 ( 2013 .01 ) ; A61K 39 / 3955 ANTIBODIES ( 2013 .01 ) ; A61K 31 /4706 ( 2013 .01 ) ; A61K 31 / 165 ( 2013 .01 ) ; CO7K 2317 /21 (2013 . 01 ) ; (71 ) Applicant: Eli D Ehrenpreis , Skokie , IL (US ) CO7K 2317/ 24 ( 2013. 01 ) ; A61K 2039/ 505 ( 2013 .01 ) (72 ) Inventor : Eli D Ehrenpreis, Skokie , IL (US ) (57 ) ABSTRACT Disclosed are methods for enhancing the efficacy of mono (21 ) Appl. No. : 15 /605 ,212 clonal antibody therapy , which entails co - administering a therapeutic monoclonal antibody , or a functional fragment (22 ) Filed : May 25 , 2017 thereof, and an effective amount of colchicine or hydroxy chloroquine , or a combination thereof, to a patient in need Related U . S . Application Data thereof . Also disclosed are methods of prolonging or increasing the time a monoclonal antibody remains in the (63 ) Continuation - in - part of application No . 14 / 947 , 193 , circulation of a patient, which entails co - administering a filed on Nov. 20 , 2015 . therapeutic monoclonal antibody , or a functional fragment ( 60 ) Provisional application No . 62/ 082, 682 , filed on Nov . of the monoclonal antibody , and an effective amount of 21 , 2014 . colchicine or hydroxychloroquine , or a combination thereof, to a patient in need thereof, wherein the time themonoclonal antibody remains in the circulation ( e . g . , blood serum ) of the Publication Classification patient is increased relative to the same regimen of admin (51 ) Int . -

Antibodies to Watch in 2021 Hélène Kaplona and Janice M
MABS 2021, VOL. 13, NO. 1, e1860476 (34 pages) https://doi.org/10.1080/19420862.2020.1860476 PERSPECTIVE Antibodies to watch in 2021 Hélène Kaplona and Janice M. Reichert b aInstitut De Recherches Internationales Servier, Translational Medicine Department, Suresnes, France; bThe Antibody Society, Inc., Framingham, MA, USA ABSTRACT ARTICLE HISTORY In this 12th annual installment of the Antibodies to Watch article series, we discuss key events in antibody Received 1 December 2020 therapeutics development that occurred in 2020 and forecast events that might occur in 2021. The Accepted 1 December 2020 coronavirus disease 2019 (COVID-19) pandemic posed an array of challenges and opportunities to the KEYWORDS healthcare system in 2020, and it will continue to do so in 2021. Remarkably, by late November 2020, two Antibody therapeutics; anti-SARS-CoV antibody products, bamlanivimab and the casirivimab and imdevimab cocktail, were cancer; COVID-19; Food and authorized for emergency use by the US Food and Drug Administration (FDA) and the repurposed Drug Administration; antibodies levilimab and itolizumab had been registered for emergency use as treatments for COVID-19 European Medicines Agency; in Russia and India, respectively. Despite the pandemic, 10 antibody therapeutics had been granted the immune-mediated disorders; first approval in the US or EU in 2020, as of November, and 2 more (tanezumab and margetuximab) may Sars-CoV-2 be granted approvals in December 2020.* In addition, prolgolimab and olokizumab had been granted first approvals in Russia and cetuximab saratolacan sodium was first approved in Japan. The number of approvals in 2021 may set a record, as marketing applications for 16 investigational antibody therapeutics are already undergoing regulatory review by either the FDA or the European Medicines Agency. -

Second-Line FOLFIRI Plus Ramucirumab with Or Without Prior
Cancer Chemotherapy and Pharmacology (2019) 84:307–313 https://doi.org/10.1007/s00280-019-03855-w ORIGINAL ARTICLE Second‑line FOLFIRI plus ramucirumab with or without prior bevacizumab for patients with metastatic colorectal cancer Takeshi Suzuki1,2 · Eiji Shinozaki1 · Hiroki Osumi1 · Izuma Nakayama1 · Yumiko Ota1 · Takashi Ichimura1 · Mariko Ogura1 · Takeru Wakatsuki1 · Akira Ooki1 · Daisuke Takahari1 · Mitsukuni Suenaga1 · Keisho Chin1 · Kensei Yamaguchi1 Received: 13 February 2019 / Accepted: 2 May 2019 / Published online: 7 May 2019 © Springer-Verlag GmbH Germany, part of Springer Nature 2019 Abstract Purpose Few data of folinic acid, fuorouracil, and irinotecan (FOLFIRI) plus ramucirumab (RAM) obtained in bevacizumab- naïve patients in clinical trials or routine clinical practice are available. The purpose of this retrospective study was to report the results of FOLFIRI plus RAM treatment as second-line chemotherapy for metastatic colorectal cancer (mCRC). Methods Seventy-four patients with mCRC who received second-line FOLFIRI + RAM mCRC therapy were stratifed by previous frst-line therapy to groups that had (PB) or had not (NPB) been given bevacizumab. The overall survival (OS), progression-free survival (PFS), and objective response were evaluated. Results The overall median PFS was 6.2 months (95% CI 4.6–9.3) and median OS was 17.0 months (95% CI 11.6–NA). Median PFS was 8.0 months (95% CI 4.9–11.2) in NPB patients and 5.0 months (95% CI 3.1–7.3) in PB patients (hazard ratio = 0.72, 95% CI 0.40–1.30, p = 0.28). The response rates were 23% and 3% in NPB and PB patients, respectively. -

In This Issue
IN THIS ISSUE PD-1 Blockade Is Not Unsafe in Patients with Lung Cancer and COVID-19 • COVID-19 severity and mortality were not • Although PD-1 blockade was not a • This suggests that PD-1 blockade increased in patients with lung cancer risk factor in this population, severity should be used when indicated in patients who received prior PD-1 blockade . and mortality in this group were high . with lung cancer despite COVID-19 . A key issue in oncology of whether patients received the immunotherapy recently or practice during the COVID-19 at any point prior to infection. There was a slight, statistically pandemic is whether PD-1 insignifi cant numeric increase in severity with PD-1 blockade, blockade affects the severity of but controlling for smoking history—which is an expected COVID-19 in patients with can- imbalance in receipt of prior PD-1 blockade and a risk factor cer. PD-1 blockade may increase for severe COVID-19 outcomes—abolished this correlation. COVID-19 severity by contrib- This work suggests that prior PD-1 blockade is not a clinically uting to hyperactive immune meaningful risk factor for worsened COVID-19 outcomes, res ponses to SARS-CoV-2 infec- implying that PD-1 blockade should be used when indicated tion—or, alternatively, may reduce despite the pandemic. Larger studies are needed to more fully severity by enhancing control of initial viral infection. To examine this question. Finally, it should be noted that, in minimize lung cancer as a confounder, Luo and colleagues this study population, more than half of patients with lung assessed the outcomes of concurrent COVID-19 and lung cancer and COVID-19 were hospitalized and almost half of cancer in 69 consecutive patients at a single institution in those hospitalized died, emphasizing the need for studies to New York City. -

Cyramza, INN-Ramucirumab
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Cyramza 10 mg/ml concentrate for solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One ml of concentrate for solution for infusion contains 10 mg ramucirumab. Each 10 ml vial contains 100 mg of ramucirumab. Each 50 ml vial contains 500 mg of ramucirumab. Ramucirumab is a human IgG1 monoclonal antibody produced in murine (NS0) cells by recombinant DNA technology. Excipient with known effect Each 10 ml vial contains approximately 17 mg sodium. Each 50 ml vial contains approximately 85 mg sodium. For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Concentrate for solution for infusion (sterile concentrate). The concentrate is a clear to slightly opalescent and colourless to slightly yellow solution, pH 6.0. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Gastric cancer Cyramza in combination with paclitaxel is indicated for the treatment of adult patients with advanced gastric cancer or gastro-oesophageal junction adenocarcinoma with disease progression after prior platinum and fluoropyrimidine chemotherapy (see section 5.1). Cyramza monotherapy is indicated for the treatment of adult patients with advanced gastric cancer or gastro-oesophageal junction adenocarcinoma with disease progression after prior platinum or fluoropyrimidine chemotherapy, for whom treatment in combination with paclitaxel is not appropriate (see section 5.1). Colorectal cancer Cyramza, in combination with FOLFIRI (irinotecan, folinic acid, and 5-fluorouracil), is indicated for the treatment of adult patients with metastatic colorectal cancer (mCRC) with disease progression on or after prior therapy with bevacizumab, oxaliplatin and a fluoropyrimidine. 2 Non-small cell lung cancer Cyramza in combination with erlotinib is indicated for the first-line treatment of adult patients with metastatic non-small cell lung cancer with activating epidermal growth factor receptor (EGFR) mutations (see section 5.1).