Microsporidioses E Mixosporidioses Da Ictiofauna
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Deep-Sea Cymothoid Isopods (Crustacea: Isopoda: Cymothoidae) of Pacifi C Coast of Northern Honshu, Japan
Deep-sea Fauna and Pollutants off Pacifi c Coast of Northern Japan, edited by T. Fujita, National Museum of Nature and Science Monographs, No. 39, pp. 467-481, 2009 Deep-sea Cymothoid Isopods (Crustacea: Isopoda: Cymothoidae) of Pacifi c Coast of Northern Honshu, Japan Takeo Yamauchi Toyama Institute of Health, 17̶1 Nakataikoyama, Imizu, Toyama, 939̶0363 Japan E-mail: [email protected] Abstract: During the project “Research on Deep-sea Fauna and Pollutants off Pacifi c Coast of Northern Ja- pan”, a small collection of cymothoid isopods was obtained at depths ranging from 150 to 908 m. Four species of cymothoid isopods including a new species are reported. Mothocya komatsui sp. nov. is distinguished from its congeners by the elongate body shape and the heavily twisting of the body. Three species, Ceratothoa oxyrrhynchaena Koelbel, 1878, Elthusa sacciger (Richardson, 1909), and Pleopodias diaphus Avdeev, 1975 were fully redescribed. Ceratothoa oxyrrhynchaena and E. sacciger were fi rstly collected from blackthroat seaperchs Doederleinia berycoides (Hilgendorf) and Kaup’s arrowtooth eels Synaphobranchus kaupii John- son, respectively. Key words: Ceratothoa oxyrrhynchaena, Elthusa sacciger, Mothocya, new host record, new species, Pleo- podias diaphus, redescription. Introduction Cymothoid isopods are ectoparasites of marine, fresh, and brackish water fi sh. In Japan, about 45 species of cymothoid isopods are known (Saito et al., 2000), but deep-sea species have not been well studied. This paper deals with a collection of cymothoid isopods from the project “Research on Deep-sea Fauna and Pollutants off Pacifi c Coast of Northern Japan” conducted by the National Museum of Nature and Science, Tokyo. -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -
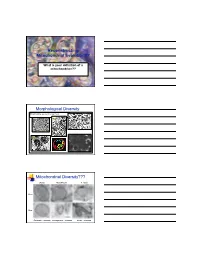
Reconstructing Mitochondrial Evolution?? Morphological Diversity Mitochondrial Diversity???
Reconstructing Mitochondrial Evolution?? What is your definition of a mitochondrion?? Morphological Diversity Mitochondria as we all know them: Suprarenal gland Liver cell Plasma cell Adrenal cortex Mitochondrial Diversity??? Chicken Neocallimastix T. foetus 100 nm 50 nm Entamoeba - mitosome microsporidian - mitosome Giardia - mitosome Reconstructing Evolution Mitochondrial evolution well established endosymbiotic theory α-proteobacterium - Rickettsia prowazekii Hydrogenosomal evolution No DNA NOW 2 examples Nyctotherus and Blastocystis (MLO) Several proteins similar to mitochondria Mitosome evolution No DNA Few proteins identified similar to mitochondria Origins via Endosymbiosis Current dogma - mitochondria and related Aerobic α-proteobacterium prokaryote gave rise to present day mitochondria. organelles arose just once in evolution Are hydrogenosomes and mitosomes of anaerobic protists derived from the same proto-mitochondrion? Evidence for: accumulating evidence for several proteins that are currently found in mitochondria - Proteins of Fe-S cluster formation. Scenario A Common ancestor facultative Scenario B organism? degenerate mitochondrion invoke lateral gene transfer from anaerobic prokaryotes Hypotheses for Mito Acquisition Mitochondrion-related Organelles 2 way to interpret this summary - can you think of both? Groups with mitochondrial homologues Hjort et al Phil. Trans. R. Soc. B 2010 365, 713-727 Origin of Hydrogenosomes (Mitosomes?) 1. a)Conversion of Mitochondria HYD b)Common ancestor with mitochondria ? HYD 2. Independent Origin α-proteobacterium ? - anaerobic bacterium HYD Organelles - origins and biogenesis Approaches: (1) Conduct phylogenetic analyses of similar proteins Hsp70 Fd Hsp60 Isc subunits (2) Examine protein targeting to the organelle matrix protein targeting membrane protein targeting (3) Characterize membrane/translocation components These components could have evolved as the endosymbiont was converted to organelle. Reveals evolutionary history. -

Redalyc.Kudoa Spp. (Myxozoa, Multivalvulida) Parasitizing Fish Caught in Aracaju, Sergipe, Brazil
Revista Brasileira de Parasitologia Veterinária ISSN: 0103-846X [email protected] Colégio Brasileiro de Parasitologia Veterinária Brasil Costa Eiras, Jorge; Yudi Fujimoto, Rodrigo; Riscala Madi, Rubens; Sierpe Jeraldo, Veronica de Lourdes; Moura de Melo, Cláudia; dos Santos de Souza, Jônatas; Picanço Diniz, José Antonio; Guerreiro Diniz, Daniel Kudoa spp. (Myxozoa, Multivalvulida) parasitizing fish caught in Aracaju, Sergipe, Brazil Revista Brasileira de Parasitologia Veterinária, vol. 25, núm. 4, octubre-diciembre, 2016, pp. 429-434 Colégio Brasileiro de Parasitologia Veterinária Jaboticabal, Brasil Available in: http://www.redalyc.org/articulo.oa?id=397848910008 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Original Article Braz. J. Vet. Parasitol., Jaboticabal, v. 25, n. 4, p. 429-434, out.-dez. 2016 ISSN 0103-846X (Print) / ISSN 1984-2961 (Electronic) Doi: http://dx.doi.org/10.1590/S1984-29612016059 Kudoa spp. (Myxozoa, Multivalvulida) parasitizing fish caught in Aracaju, Sergipe, Brazil Kudoa spp. (Myxozoa, Multivalvulida) parasitando peixes capturados em Aracaju, Sergipe, Brasil Jorge Costa Eiras1; Rodrigo Yudi Fujimoto2; Rubens Riscala Madi3; Veronica de Lourdes Sierpe Jeraldo4; Cláudia Moura de Melo4; Jônatas dos Santos de Souza5; José Antonio Picanço Diniz6; Daniel Guerreiro Diniz7* -

A Dissertation Entitled Evolution, Systematics
A Dissertation Entitled Evolution, systematics, and phylogeography of Ponto-Caspian gobies (Benthophilinae: Gobiidae: Teleostei) By Matthew E. Neilson Submitted as partial fulfillment of the requirements for The Doctor of Philosophy Degree in Biology (Ecology) ____________________________________ Adviser: Dr. Carol A. Stepien ____________________________________ Committee Member: Dr. Christine M. Mayer ____________________________________ Committee Member: Dr. Elliot J. Tramer ____________________________________ Committee Member: Dr. David J. Jude ____________________________________ Committee Member: Dr. Juan L. Bouzat ____________________________________ College of Graduate Studies The University of Toledo December 2009 Copyright © 2009 This document is copyrighted material. Under copyright law, no parts of this document may be reproduced without the expressed permission of the author. _______________________________________________________________________ An Abstract of Evolution, systematics, and phylogeography of Ponto-Caspian gobies (Benthophilinae: Gobiidae: Teleostei) Matthew E. Neilson Submitted as partial fulfillment of the requirements for The Doctor of Philosophy Degree in Biology (Ecology) The University of Toledo December 2009 The study of biodiversity, at multiple hierarchical levels, provides insight into the evolutionary history of taxa and provides a framework for understanding patterns in ecology. This is especially poignant in invasion biology, where the prevalence of invasiveness in certain taxonomic groups could -

Pdf (595.62 K)
Provided for non-commercial research and education use. Not for reproduction, distribution or commercial use. Vol. 10 No. 1 (2018) Egyptian Academic Journal of Biological Sciences is the official English language journal of the Egyptian Society of Biological Sciences, Department of Entomology, Faculty of Sciences Ain Shams University. The Journal publishes original research papers and reviews from any zoological discipline or from directly allied fields in ecology, behavioral biology, physiology & biochemistry. www.eajbs.eg.net Citation: Egypt. Acad. J. Biolog. Sci. (B. Zoology) Vol. 10(1)pp1-17 (2018) Egypt. Acad. J. Biolog. Sci., 10(1): 1- 17 (2018) Egyptian Academic Journal of Biological Sciences B. Zoology ISSN: 2090 – 0759 www.eajbs.eg.net Infestation Study of Livoneca redmanii (Isopoda, Cymothoidae) on Mugil cephalus in Lake Qarun, Egypt Ahmed M. Helal and Osama E. A. Yousef Marine Biology branch, Zoology Depart., Faculty of Science, Al-Azhar University, Cairo, Egypt E.Mail :[email protected] ARTICLE INFO ABSTRACT Article History The present study deals with the infestation study of Received:25/12/2017 Livoneca redmanii (Isopoda, Cymothoidae) on Mugil cephalus in Accepted: 16/1/2018 Lake Qarun, Egypt. Out of 576 examined fish collected monthly _________________ from the different localities of Lake Qarun during the period Keywords: (January - December, 2016) there are 269 (46.7 %) fish were Infestation, Livoneca infested by crustacean parasite. Results showed that the highest redmanii, infestation percentage in Mugil cephalus was 76.20 % recorded in Histopathology, Mugil February and the lowest one occurred in January (19.40 %). cephalus, Lake Qarun Generally, the infestation percentage in male fishes is lower than those in females being, 34.6 % and 65.4 % respectively. -

The Round Goby (Neogobius Melanostomus):A Review of European and North American Literature
ILLINOI S UNIVERSITY OF ILLINOIS AT URBANA-CHAMPAIGN PRODUCTION NOTE University of Illinois at Urbana-Champaign Library Large-scale Digitization Project, 2007. CI u/l Natural History Survey cF Library (/4(I) ILLINOIS NATURAL HISTORY OT TSrX O IJX6V E• The Round Goby (Neogobius melanostomus):A Review of European and North American Literature with notes from the Round Goby Conference, Chicago, 1996 Center for Aquatic Ecology J. Ei!en Marsden, Patrice Charlebois', Kirby Wolfe Illinois Natural History Survey and 'Illinois-Indiana Sea Grant Lake Michigan Biological Station 400 17th St., Zion IL 60099 David Jude University of Michigan, Great Lakes Research Division 3107 Institute of Science & Technology Ann Arbor MI 48109 and Svetlana Rudnicka Institute of Fisheries Varna, Bulgaria Illinois Natural History Survey Lake Michigan Biological Station 400 17th Sti Zion, Illinois 6 Aquatic Ecology Technical Report 96/10 The Round Goby (Neogobius melanostomus): A Review of European and North American Literature with Notes from the Round Goby Conference, Chicago, 1996 J. Ellen Marsden, Patrice Charlebois1, Kirby Wolfe Illinois Natural History Survey and 'Illinois-Indiana Sea Grant Lake Michigan Biological Station 400 17th St., Zion IL 60099 David Jude University of Michigan, Great Lakes Research Division 3107 Institute of Science & Technology Ann Arbor MI 48109 and Svetlana Rudnicka Institute of Fisheries Varna, Bulgaria The Round Goby Conference, held on Feb. 21-22, 1996, was sponsored by the Illinois-Indiana Sea Grant Program, and organized by the -

ARIID Ariu 29 1983 FAO SPECIES IDENTIFICATION SHEETS FAMILY
click for previous page ARIID Ariu 29 1983 FAO SPECIES IDENTIFICATION SHEETS FAMILY: ARIIDAE FISHING AREA 51 (W. Indian Ocean) Arius sumatranus Bennett, 1830 OTHER SCIENTIFIC NAMES STILL IN USE: Tachysurus sumatranus (Bennett, 1830) VERNACULAR NAMES: FAO : En - Goat catfish Fr - Mâchoiron surmulet Sp - Bagre chivato NATIONAL : DISTINCTIVE CHARACTERS: Dorsal profile of head as a gentle slope to first dorsal fin base; 3 pairs of barbels around mouth, the maxillary pair extending to pectoral fin base; head shield thinly granulated and rugose on occipital region only; supraoccipital process keeled, as long as broad at base, its hind end emarginate; median longitudinal groove narrow, reaching to base of supraoccipital process; predorsal plate U-shaped; premaxillary band of teeth in upper jaw 5 times as long as broad, mandibulary band of teeth in lower jaw deeply curved and medially interrupted, palate teeth villïform, in a single ovate patch on each side, sparsely packed. and well separated. First dorsal and pectoral fins each with a strong spine; total anal fin rays 18 to 23. Colour: dark brown above becoming lighter on sides and on belly; fins grey-edged, adipose fin greyish. DISTINGUISHING CHARACTERS OF SIMILAR SPECIES OCCURRING IN THE AREA: snout long Arius caelatus: palate tooth patches much and spatulate larger than in A. sumatranus, triangular and densely maxillary packed. barbel short A. platystomus: palate tooth patches large, snout duck-bill shaped oval, and more densely packed; snout duck-bill shaped. A. subrostratus: palate tooth patches with densely packed teeth; snout long, spatulate, maxil- lary barbels usually not extending beyond eye. A. arius, A. -

Unesco-Eolss Sample Chapters
FISHERIES AND AQUACULTURE - Myxozoan Biology And Ecology - Dr. Ariadna Sitjà-Bobadilla and Oswaldo Palenzuela MYXOZOAN BIOLOGY AND ECOLOGY Ariadna Sitjà-Bobadilla and Oswaldo Palenzuela Instituto de Acuicultura Torre de la Sal, Consejo Superior de Investigaciones Científicas (IATS-CSIC), Castellón, Spain Keywords: Myxozoa, Myxosporea, Actinosporea, Malacosporea, Metazoa, Parasites, Fish Pathology, Invertebrates, Taxonomy, Phylogeny, Cell Biology, Life Cycle Contents 1. Introduction 2. Phylogeny 3. Morphology and Taxonomy 3.1. Spore Morphology 3.2. Taxonomy 4. Life Cycle 4.1. Life Cycle of Myxosporea 4.2. Life Cycle of Malacosporea 5. Cell Biology and Development 6. Ecological Aspects 6.1. Hosts 6.2. Habitats 6.3. Environmental Cues 7. Pathology 7.1. General Remarks 7.2. Pathogenic Effects of Myxozoans 7.2.1. Effects on Invertebrates 7.2.2. Effects on Fish 7.2.3. Effects on non-fish Vertebrates Acknowledgements Glossary Bibliography Biographical Sketches Summary UNESCO-EOLSS The phylum Myxozoa is a group of microscopic metazoans with an obligate endoparasitic lifestyle.SAMPLE Traditionally regarded CHAPTERS as protists, research findings during the last decades have dramatically changed our knowledge of these organisms, nowadays understood as examples of early metazoan evolution and extreme adaptation to parasitic lifestyles. Two distinct classes of myxozoans, Myxosporea and Malacosporea, are characterized by profound differences in rDNA evolution and well supported by differential biological and developmental features. This notwithstanding, most of the existing Myxosporea subtaxa require revision in the light of molecular phylogeny data. Most known myxozoans exhibit diheteroxenous cycles, alternating between a vertebrate host (mostly fish but also other poikilothermic vertebrates, and exceptionally birds and mammals) and an invertebrate (mainly annelids and bryozoans but possibly other ©Encyclopedia of Life Support Systems (EOLSS) FISHERIES AND AQUACULTURE - Myxozoan Biology And Ecology - Dr. -

Fish Diversity and Habitat Relationship with Environmental Variables at Meghna River Estuary, Bangladesh
Egyptian Journal of Aquatic Research (2012) 38, 213–226 National Institute of Oceanography and Fisheries Egyptian Journal of Aquatic Research http://ees.elsevier.com/ejar www.sciencedirect.com FULL LENGTH ARTICLE Fish diversity and habitat relationship with environmental variables at Meghna river estuary, Bangladesh M. Shahadat Hossain, Nani Gopal Das, Subrata Sarker *, M. Ziaur Rahaman Institute of Marine Sciences and Fisheries, University of Chittagong, Chittagong 4331, Bangladesh Received 26 November 2012; accepted 7 December 2012 Available online 8 February 2013 KEYWORDS Abstract Meghna river estuary is the largest estuarine ecosystem of Bangladesh and support Fish diversity; diverse fisheries communities compared to others. Present study was carried out to assess the fish Habitat; diversity status with relation to major hydrological and meteorological parameters in both spa- Environment; tio-temporal scales. Fish samples were collected together with water quality parameters from eight Meghna estuary sampling stations of the Meghna river estuary from November 2011 to April 2012. Fifty years mete- orological data were collected from meteorological department. Diversity status were analyzed from all fisheries data by using PAST (version 2.15) software. Findings showed that Meghna river estuary is the habitat of 53 fish species and Oxyurichthys microlepis, Hemiarius sona Arius thalassinus, Batrachocephalus mino and Arius caelatus are the major contributory species (>6%) for both spa- tio-temporal scales. Water temperature and rainfall was -

Marine Fishes of the Azores: an Annotated Checklist and Bibliography
MARINE FISHES OF THE AZORES: AN ANNOTATED CHECKLIST AND BIBLIOGRAPHY. RICARDO SERRÃO SANTOS, FILIPE MORA PORTEIRO & JOÃO PEDRO BARREIROS SANTOS, RICARDO SERRÃO, FILIPE MORA PORTEIRO & JOÃO PEDRO BARREIROS 1997. Marine fishes of the Azores: An annotated checklist and bibliography. Arquipélago. Life and Marine Sciences Supplement 1: xxiii + 242pp. Ponta Delgada. ISSN 0873-4704. ISBN 972-9340-92-7. A list of the marine fishes of the Azores is presented. The list is based on a review of the literature combined with an examination of selected specimens available from collections of Azorean fishes deposited in museums, including the collection of fish at the Department of Oceanography and Fisheries of the University of the Azores (Horta). Personal information collected over several years is also incorporated. The geographic area considered is the Economic Exclusive Zone of the Azores. The list is organised in Classes, Orders and Families according to Nelson (1994). The scientific names are, for the most part, those used in Fishes of the North-eastern Atlantic and the Mediterranean (FNAM) (Whitehead et al. 1989), and they are organised in alphabetical order within the families. Clofnam numbers (see Hureau & Monod 1979) are included for reference. Information is given if the species is not cited for the Azores in FNAM. Whenever available, vernacular names are presented, both in Portuguese (Azorean names) and in English. Synonyms, misspellings and misidentifications found in the literature in reference to the occurrence of species in the Azores are also quoted. The 460 species listed, belong to 142 families; 12 species are cited for the first time for the Azores. -

Atlas of North Sea Fishes
ICES COOPERATIVE RESEARCH REPORT RAPPORT DES RECHERCHES COLLECTIVES NO. 194 Atlas of North Sea Fishes Based on bottom-trawl survey data for the years 1985—1987 Ruud J. Knijn1, Trevor W. Boon2, Henk J. L. Heessen1, and John R. G. Hislop3 'Netherlands Institute for Fisheries Research, Haringkade 1, PO Box 6 8 , 1970 AB Umuiden, The Netherlands 2MAFF, Fisheries Laboratory, Lowestoft, Suffolk NR33 OHT, England 3Marine Laboratory, PO Box 101, Victoria Road, Aberdeen AB9 8 DB, Scotland Fish illustrations by Peter Stebbing International Council for the Exploration of the Sea Conseil International pour l’Exploration de la Mer Palægade 2—4, DK-1261 Copenhagen K, Denmark September 1993 Copyright ® 1993 All rights reserved No part of this book may be reproduced in any form by photostat or microfilm or stored in a storage system or retrieval system or by any other means without written permission from the authors and the International Council for the Exploration of the Sea Illustrations ® 1993 Peter Stebbing Published with financial support from the Directorate-General for Fisheries, AIR Programme, of the Commission of the European Communities ICES Cooperative Research Report No. 194 Atlas of North Sea Fishes ISSN 1017-6195 Printed in Denmark Contents 1. Introduction............................................................................................................... 1 2. Recruit surveys.................................................................................. 3 2.1 General purpose of the surveys.....................................................................