The Short Third Intracellular Loop and Cytoplasmic Tail of Bitter Taste
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Looking for New Classes of Bronchodilators
REVIEW BRONCHODILATORS The future of bronchodilation: looking for new classes of bronchodilators Mario Cazzola1, Paola Rogliani 1 and Maria Gabriella Matera2 Affiliations: 1Dept of Experimental Medicine, University of Rome Tor Vergata, Rome, Italy. 2Dept of Experimental Medicine, University of Campania “Luigi Vanvitelli”, Naples, Italy. Correspondence: Mario Cazzola, Dept of Experimental Medicine, University of Rome Tor Vergata, Via Montpellier 1, Rome, 00133, Italy. E-mail: [email protected] @ERSpublications There is a real interest among researchers and the pharmaceutical industry in developing novel bronchodilators. There are several new opportunities; however, they are mostly in a preclinical phase. They could better optimise bronchodilation. http://bit.ly/2lW1q39 Cite this article as: Cazzola M, Rogliani P, Matera MG. The future of bronchodilation: looking for new classes of bronchodilators. Eur Respir Rev 2019; 28: 190095 [https://doi.org/10.1183/16000617.0095-2019]. ABSTRACT Available bronchodilators can satisfy many of the needs of patients suffering from airway disorders, but they often do not relieve symptoms and their long-term use raises safety concerns. Therefore, there is interest in developing new classes that could help to overcome the limits that characterise the existing classes. At least nine potential new classes of bronchodilators have been identified: 1) selective phosphodiesterase inhibitors; 2) bitter-taste receptor agonists; 3) E-prostanoid receptor 4 agonists; 4) Rho kinase inhibitors; 5) calcilytics; 6) agonists of peroxisome proliferator-activated receptor-γ; 7) agonists of relaxin receptor 1; 8) soluble guanylyl cyclase activators; and 9) pepducins. They are under consideration, but they are mostly in a preclinical phase and, consequently, we still do not know which classes will actually be developed for clinical use and whether it will be proven that a possible clinical benefit outweighs the impact of any adverse effect. -

Modulation of Food Intake by Differential TAS2R Stimulation In
nutrients Article Modulation of Food Intake by Differential TAS2R Stimulation in Rat Carme Grau-Bové 1, Alba Miguéns-Gómez 1 , Carlos González-Quilen 1 , José-Antonio Fernández-López 2,3 , Xavier Remesar 2,3 , Cristina Torres-Fuentes 4 , Javier Ávila-Román 4 , Esther Rodríguez-Gallego 1, Raúl Beltrán-Debón 1 , M Teresa Blay 1 , Ximena Terra 1 , Anna Ardévol 1,* and Montserrat Pinent 1 1 MoBioFood Research Group, Department of Biochemistry and Biotechnology, Universitat Rovira i Virgili, 43007 Tarragona, Spain; [email protected] (C.G.-B.); [email protected] (A.M.-G.); [email protected] (C.G.-Q.); [email protected] (E.R.-G.); [email protected] (R.B.-D.); [email protected] (M.T.B.); [email protected] (X.T.); [email protected] (M.P.) 2 Department of Biochemistry and Molecular Biomedicine, Faculty of Biology, University of Barcelona, Av. Diagonal 643, 08028 Barcelona, Spain; [email protected] (J.-A.F.-L.); [email protected] (X.R.) 3 CIBER Obesity and Nutrition, Institute of Health Carlos III, Av. Diagonal 643, 08028 Barcelona, Spain 4 Nutrigenomics Research Group, Department of Biochemistry and Biotechnology, Universitat Rovira i Virgili, 43007 Tarragona, Spain; [email protected] (C.T.-F.); [email protected] (J.Á.-R.) * Correspondence: [email protected]; Tel.: +34-977-559-566 Received: 31 October 2020; Accepted: 4 December 2020; Published: 10 December 2020 Abstract: Metabolic surgery modulates the enterohormone profile, which leads, among other effects, to changes in food intake. Bitter taste receptors (TAS2Rs) have been identified in the gastrointestinal tract and specific stimulation of these has been linked to the control of ghrelin secretion. -

G Protein-Coupled Receptors
S.P.H. Alexander et al. The Concise Guide to PHARMACOLOGY 2015/16: G protein-coupled receptors. British Journal of Pharmacology (2015) 172, 5744–5869 THE CONCISE GUIDE TO PHARMACOLOGY 2015/16: G protein-coupled receptors Stephen PH Alexander1, Anthony P Davenport2, Eamonn Kelly3, Neil Marrion3, John A Peters4, Helen E Benson5, Elena Faccenda5, Adam J Pawson5, Joanna L Sharman5, Christopher Southan5, Jamie A Davies5 and CGTP Collaborators 1School of Biomedical Sciences, University of Nottingham Medical School, Nottingham, NG7 2UH, UK, 2Clinical Pharmacology Unit, University of Cambridge, Cambridge, CB2 0QQ, UK, 3School of Physiology and Pharmacology, University of Bristol, Bristol, BS8 1TD, UK, 4Neuroscience Division, Medical Education Institute, Ninewells Hospital and Medical School, University of Dundee, Dundee, DD1 9SY, UK, 5Centre for Integrative Physiology, University of Edinburgh, Edinburgh, EH8 9XD, UK Abstract The Concise Guide to PHARMACOLOGY 2015/16 provides concise overviews of the key properties of over 1750 human drug targets with their pharmacology, plus links to an open access knowledgebase of drug targets and their ligands (www.guidetopharmacology.org), which provides more detailed views of target and ligand properties. The full contents can be found at http://onlinelibrary.wiley.com/doi/ 10.1111/bph.13348/full. G protein-coupled receptors are one of the eight major pharmacological targets into which the Guide is divided, with the others being: ligand-gated ion channels, voltage-gated ion channels, other ion channels, nuclear hormone receptors, catalytic receptors, enzymes and transporters. These are presented with nomenclature guidance and summary information on the best available pharmacological tools, alongside key references and suggestions for further reading. -

Multi-Functionality of Proteins Involved in GPCR and G Protein Signaling: Making Sense of Structure–Function Continuum with In
Cellular and Molecular Life Sciences (2019) 76:4461–4492 https://doi.org/10.1007/s00018-019-03276-1 Cellular andMolecular Life Sciences REVIEW Multi‑functionality of proteins involved in GPCR and G protein signaling: making sense of structure–function continuum with intrinsic disorder‑based proteoforms Alexander V. Fonin1 · April L. Darling2 · Irina M. Kuznetsova1 · Konstantin K. Turoverov1,3 · Vladimir N. Uversky2,4 Received: 5 August 2019 / Revised: 5 August 2019 / Accepted: 12 August 2019 / Published online: 19 August 2019 © Springer Nature Switzerland AG 2019 Abstract GPCR–G protein signaling system recognizes a multitude of extracellular ligands and triggers a variety of intracellular signal- ing cascades in response. In humans, this system includes more than 800 various GPCRs and a large set of heterotrimeric G proteins. Complexity of this system goes far beyond a multitude of pair-wise ligand–GPCR and GPCR–G protein interactions. In fact, one GPCR can recognize more than one extracellular signal and interact with more than one G protein. Furthermore, one ligand can activate more than one GPCR, and multiple GPCRs can couple to the same G protein. This defnes an intricate multifunctionality of this important signaling system. Here, we show that the multifunctionality of GPCR–G protein system represents an illustrative example of the protein structure–function continuum, where structures of the involved proteins represent a complex mosaic of diferently folded regions (foldons, non-foldons, unfoldons, semi-foldons, and inducible foldons). The functionality of resulting highly dynamic conformational ensembles is fne-tuned by various post-translational modifcations and alternative splicing, and such ensembles can undergo dramatic changes at interaction with their specifc partners. -

G Protein‐Coupled Receptors
S.P.H. Alexander et al. The Concise Guide to PHARMACOLOGY 2019/20: G protein-coupled receptors. British Journal of Pharmacology (2019) 176, S21–S141 THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: G protein-coupled receptors Stephen PH Alexander1 , Arthur Christopoulos2 , Anthony P Davenport3 , Eamonn Kelly4, Alistair Mathie5 , John A Peters6 , Emma L Veale5 ,JaneFArmstrong7 , Elena Faccenda7 ,SimonDHarding7 ,AdamJPawson7 , Joanna L Sharman7 , Christopher Southan7 , Jamie A Davies7 and CGTP Collaborators 1School of Life Sciences, University of Nottingham Medical School, Nottingham, NG7 2UH, UK 2Monash Institute of Pharmaceutical Sciences and Department of Pharmacology, Monash University, Parkville, Victoria 3052, Australia 3Clinical Pharmacology Unit, University of Cambridge, Cambridge, CB2 0QQ, UK 4School of Physiology, Pharmacology and Neuroscience, University of Bristol, Bristol, BS8 1TD, UK 5Medway School of Pharmacy, The Universities of Greenwich and Kent at Medway, Anson Building, Central Avenue, Chatham Maritime, Chatham, Kent, ME4 4TB, UK 6Neuroscience Division, Medical Education Institute, Ninewells Hospital and Medical School, University of Dundee, Dundee, DD1 9SY, UK 7Centre for Discovery Brain Sciences, University of Edinburgh, Edinburgh, EH8 9XD, UK Abstract The Concise Guide to PHARMACOLOGY 2019/20 is the fourth in this series of biennial publications. The Concise Guide provides concise overviews of the key properties of nearly 1800 human drug targets with an emphasis on selective pharmacology (where available), plus links to the open access knowledgebase source of drug targets and their ligands (www.guidetopharmacology.org), which provides more detailed views of target and ligand properties. Although the Concise Guide represents approximately 400 pages, the material presented is substantially reduced compared to information and links presented on the website. -

Bivariate Genome-Wide Association Analysis Strengthens the Role of Bitter Receptor Clusters on Chromosomes 7 and 12 in Human Bitter Taste
bioRxiv preprint doi: https://doi.org/10.1101/296269; this version posted April 6, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Bivariate genome-wide association analysis strengthens the role of bitter receptor clusters on chromosomes 7 and 12 in human bitter taste Liang-Dar Hwang1,2,3,4, Puya Gharahkhani1, Paul A. S. Breslin5,6, Scott D. Gordon1, Gu Zhu1, Nicholas G. Martin1, Danielle R. Reed5, and Margaret J. Wright2,7 1 QIMR Berghofer Medical Research Institute, Herston, Queensland 4006, Australia 2 Queensland Brain Institute, University of Queensland, St Lucia, Queensland 4072, Australia 3 Faculty of Medicine, University of Queensland, Herston, Queensland 4006, Australia 4 University of Queensland Diamantina Institute, University of Queensland, Translational Research Institute, Woolloongabba, Queensland 4102, Australia 5 Monell Chemical Senses Center, Philadelphia, Pennsylvania 19104, USA 6 Department of Nutritional Sciences, School of Environmental and Biological Sciences, Rutgers University, New Brunswick NJ, 08901 USA 7 Centre for Advanced Imaging, University of Queensland, St Lucia, Queensland 4072, Australia Correspondence to be sent to: Liang-Dar Hwang University of Queensland Diamantina Institute Wolloongabba QLD 4102, Australia Email: [email protected] Telephone: +61 7 3443 7976 Fax: +61 7 3443 6966 1 bioRxiv preprint doi: https://doi.org/10.1101/296269; this version posted April 6, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -

Prostaglandin E2 As Mediator and Modulator of Airway Smooth Muscle Responses
Institute of Environmental Medicine Division of Physiology The Unit for Experimental Asthma and Allergy Research Karolinska Institutet, Stockholm, Sweden PROSTAGLANDIN E2 AS MEDIATOR AND MODULATOR OF AIRWAY SMOOTH MUSCLE RESPONSES Jesper Säfholm Stockholm 2013 All published papers were reproduced with permission from the publisher. Published by Karolinska Institutet. Printed by Repro Print AB © Jesper Säfholm, 2013 ISBN 978-91-7549-167-7 Printed by 2013 Gårdsvägen 4, 169 70 Solna To boldly go where a lot of people have gone before ABSTRACT Prostaglandin E2 (PGE2) is a lipid mediator produced by virtually every cell of the human body. Because common non-steroidal anti-inflammatory drugs (NSAIDs) inhibit its biosynthesis, PGE2 is usually considered to be a ‘pro-inflammatory’ mediator. The role of PGE2 in the lung and airways has however always been unclear. In particular, the airway responses caused by activation of its four different EP receptors have been debated. Research on the mechanisms involved in the actions of PGE2 has previously been limited by the low potency and selectivity of available pharmacological tools. Recently, a number of potent receptor antagonists and enzyme inhibitors have become available. The aim of this thesis was therefore to characterise airway responses to PGE2 in greater detail, focusing on the role of its receptors on baseline smooth muscle function and during antigen-induced contractions. Alongside investigating PGE2 responses, the newly discovered relaxant effects of bitter tasting substances acting at their respective receptors (TAS2Rs) were examined. The project mainly involved analysis of isometric contractions and relaxations in isolated airways from guinea pigs and humans in organ baths. -

Transcriptome Analysis Reveals Upregulation of Bitter Taste Receptors in Severe Asthmatics
ORIGINAL ARTICLE ASTHMA Transcriptome analysis reveals upregulation of bitter taste receptors in severe asthmatics Christina Orsmark-Pietras1,2, Anna James2,3, Jon R. Konradsen2,4,5, Bjo¨rn Nordlund2,4,5, Cilla So¨derha¨ll1,2, Ville Pulkkinen2,3,6,7, Christophe Pedroletti2,5, Kameran Daham2,8,9, Maciek Kupczyk2,3, Barbro Dahle´n2,8,9, Juha Kere1,2,10, Sven-Erik Dahle´n2,3, Gunilla Hedlin2,4,5,11 and Erik Mele´n2,3,4,11 Affiliations: 1Dept of Biosciences and Nutrition at Novum, Karolinska Institute, Stockholm, 2The Centre for Allergy Research at Karolinska Institute, Stockholm, 3Institute of Environmental Medicine, Karolinska Institute, Stockholm, 4Astrid Lindgren Children’s Hospital, Karolinska University Hospital, Stockholm, 5Dept of Women’s and Children’s Health, Karolinska Institute, Stockholm, 8Lung/Allergy Clinic, Karolinska University Hospital Huddinge, Stockholm, 9Dept of Internal Medicine, Karolinska University Hospital Huddinge, Stockholm, and 10Science for Life Laboratory, Karolinska Institute, Stockholm, Sweden. 6Dept of Medical Genetics, Biomedicum Helsinki, University of Helsinki, Helsinki, and 7Folkha¨lsan Institute of Genetics, Helsinki, Finland. 11Both authors contributed equally. Correspondence: S-E. Dahle´n, The National Institute of Environmental Medicine, Karolinska Institutet, SE- 17177 Stockholm, Sweden. E-mail: [email protected] ABSTRACT The causes of severe childhood asthma are poorly understood. Our aim was to define global patterns of gene expression in children with severe therapy-resistant and controlled asthma. White blood cells were isolated and the global transcriptome profile was characterised using the Affymetrix Human Gene ST 1.0 chip in children with severe, therapy-resistant asthma (n517), controlled asthma (n519) and healthy controls (n518). -

The Bitter Taste Receptor Tas2r14 Is Expressed in Ovarian Cancer and Mediates Apoptotic Signalling
THE BITTER TASTE RECEPTOR TAS2R14 IS EXPRESSED IN OVARIAN CANCER AND MEDIATES APOPTOTIC SIGNALLING by Louis T. P. Martin Submitted in partial fulfilment of the requirements for the degree of Master of Science at Dalhousie University Halifax, Nova Scotia June 2017 © Copyright by Louis T. P. Martin, 2017 DEDICATION PAGE To my grandparents, Christina, Frank, Brenda and Bernie, and my parents, Angela and Tom – for teaching me the value of hard work. ii TABLE OF CONTENTS LIST OF TABLES ............................................................................................................. vi LIST OF FIGURES .......................................................................................................... vii ABSTRACT ....................................................................................................................... ix LIST OF ABBREVIATIONS AND SYMBOLS USED .................................................... x ACKNOWLEDGEMENTS .............................................................................................. xii CHAPTER 1 INTRODUCTION ........................................................................................ 1 1.1 G-PROTEIN COUPLED RECEPTORS ................................................................ 1 1.2 GPCR CLASSES .................................................................................................... 4 1.3 GPCR SIGNALING THROUGH G PROTEINS ................................................... 6 1.4 BITTER TASTE RECEPTORS (TAS2RS) ........................................................... -

G Protein-Coupled Receptors
Alexander, S. P. H., Christopoulos, A., Davenport, A. P., Kelly, E., Marrion, N. V., Peters, J. A., Faccenda, E., Harding, S. D., Pawson, A. J., Sharman, J. L., Southan, C., Davies, J. A. (2017). THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: G protein-coupled receptors. British Journal of Pharmacology, 174, S17-S129. https://doi.org/10.1111/bph.13878 Publisher's PDF, also known as Version of record License (if available): CC BY Link to published version (if available): 10.1111/bph.13878 Link to publication record in Explore Bristol Research PDF-document This is the final published version of the article (version of record). It first appeared online via Wiley at https://doi.org/10.1111/bph.13878 . Please refer to any applicable terms of use of the publisher. University of Bristol - Explore Bristol Research General rights This document is made available in accordance with publisher policies. Please cite only the published version using the reference above. Full terms of use are available: http://www.bristol.ac.uk/red/research-policy/pure/user-guides/ebr-terms/ S.P.H. Alexander et al. The Concise Guide to PHARMACOLOGY 2017/18: G protein-coupled receptors. British Journal of Pharmacology (2017) 174, S17–S129 THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: G protein-coupled receptors Stephen PH Alexander1, Arthur Christopoulos2, Anthony P Davenport3, Eamonn Kelly4, Neil V Marrion4, John A Peters5, Elena Faccenda6, Simon D Harding6,AdamJPawson6, Joanna L Sharman6, Christopher Southan6, Jamie A Davies6 and CGTP Collaborators 1 School of Life Sciences, -

Oxygenated Fatty Acids Enhance Hematopoiesis Via the Receptor GPR132
Oxygenated Fatty Acids Enhance Hematopoiesis via the Receptor GPR132 The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Lahvic, Jamie L. 2017. Oxygenated Fatty Acids Enhance Hematopoiesis via the Receptor GPR132. Doctoral dissertation, Harvard University, Graduate School of Arts & Sciences. Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:42061504 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA Oxygenated Fatty Acids Enhance Hematopoiesis via the Receptor GPR132 A dissertation presented by Jamie L. Lahvic to The Division of Medical Sciences in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the subject of Developmental and Regenerative Biology Harvard University Cambridge, Massachusetts May 2017 © 2017 Jamie L. Lahvic All rights reserved. Dissertation Advisor: Leonard I. Zon Jamie L. Lahvic Oxygenated Fatty Acids Enhance Hematopoiesis via the Receptor GPR132 Abstract After their specification in early development, hematopoietic stem cells (HSCs) maintain the entire blood system throughout adulthood as well as upon transplantation. The processes of HSC specification, renewal, and homing to the niche are regulated by protein, as well as lipid signaling molecules. A screen for chemical enhancers of marrow transplant in the zebrafish identified the endogenous lipid signaling molecule 11,12-epoxyeicosatrienoic acid (11,12-EET). EET has vasodilatory properties, but had no previously described function on HSCs. -
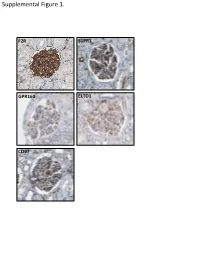
Supplemental Data
Supplemental Figure 1. F2R S1PR1 GPR160 ELTD1 CD97 Supplemental Figure 2. A B brain heart lung liver kidney spleen testis skm Expression in mouse ssues glom rok Gprc5a Gapdh Human Protein Atlas RNAseq database Supplemental Figure 3. A Gprc5a Pdgfrb Merged CL CL Gprc5a CD31 Merged CL CL B C 1.4 * 1.2 mc 1 matrix 0.8 0.6 0.4 matrix 0.2 0 pod end 1 2 mes3 D Vector Gprc5a E 40 kD - - Gprc5a - acn Supplemental Figure 4. Control 12 month-old KO 12 month-old A B C D E F 1 2 0.8 * 1.5 score 0.6 m µ 1 0.4 0.2 Slits/ 0.5 Mesangial 0 0 Ctrl KO Ctrl KO Supplemental Figure 5. A B 1.2 Vector Gprc5a 1 * 0.8 0.6 * 0.4 * Normalized Density 0.2 0 pEGFR/tEGR pSmad/tSmad TGF-β1 C 1.8 siCON 1.6 siGprc5a * 1.4 * * 1.2 1 0.8 0.6 NormalizedDensity 0.4 0.2 0 pEGFR/tEGR pSmad/tSmad TGF-β1 Supplemental table 1. List of glomerulus-expressed GPCRs as detected by qPCR. Data shown as mean ± standard deviation (Glom=glomerulus, Rok=rest of kidney). GPCR Glom Rok Glom/Rok LPAR6 41892,11 ± 38478,89 1040,06 ± 1370,12 39,28 ELTD1 30275,64 ± 14085,26 33,23 ± 46,99 910,13 GPR116 24020,06 ± 7789,84 55,1 ± 47,75 434,96 PTH1R 15402,81 ± 17644,32 7521,01 ± 3264,57 1,05 CALCRL 14096,09 ± 3854,84 199,06 ± 222,52 69,81 HPRT1 13342,04 ± 10677,69 1824,77 ± 1767,23 6,31 S1PR5 9474,7 ± 10124,3 110,84 ± 29,54 84,48 LPHN2 8645,89 ± 914,74 256,04 ± 293,87 32,77 FZD1 8176,2 ± 4947,45 1321,97 ± 1311,91 5,18 CXCR4 7097,31 ± 4388,91 535,98 ± 640,28 12,24 GPR160 6446,59 ± 1550,07 4816,3 ± 5918,99 0,34 NPY1R 6177,74 ± 6282,76 209,64 ± 296,48 28,47 PTGER4 5323,66 ± 3789,51 179,13 ± 210 28,72 RXFP1