The Relative Importance of Patch Habitat Quality and Landscape Attributes on a Declining Steppe-Bird Metapopulation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Extensión De La Banda Ancha En La Provincia De Huesca Listado De
Extensión de la banda ancha en la provincia de Huesca Listado de núcleos UNIÓN EUROPEA Extensión banda ancha en la provincia de Huesca Listado de las 321 entidades singulares de población sobre las que actuará la Diputación Provincial de Huesca dentro del Plan de Extensión de la Banda Ancha Comarca Municipio Núcleo Alto Gállego Biescas Aso de Sobremonte Alto Gállego Biescas Escuer Alto Gállego Biescas Oliván Alto Gállego Biescas Orós Alto Alto Gállego Biescas Orós Bajo Alto Gállego Biescas Yosa de Sobremonte Alto Gállego Caldearenas Anzánigo Alto Gállego Caldearenas Aquilué Alto Gállego Caldearenas Javierrelatre Alto Gállego Caldearenas Latre Alto Gállego Caldearenas San Vicente Alto Gállego Hoz de Jaca Hoz de Jaca Alto Gállego Panticosa Panticosa Alto Gállego Panticosa Pueyo de Jaca (El) Alto Gállego Sabiñánigo Artosilla Alto Gállego Sabiñánigo Aurín Alto Gállego Sabiñánigo Cartirana Alto Gállego Sabiñánigo Ibort Alto Gállego Sabiñánigo Isún de Basa Alto Gállego Sabiñánigo Lárrede Alto Gállego Sabiñánigo Larrés Alto Gállego Sabiñánigo Latas Alto Gállego Sabiñánigo Osán Alto Gállego Sabiñánigo Pardinilla Alto Gállego Sabiñánigo Puente de Sabiñánigo (El) Alto Gállego Sabiñánigo Sabiñánigo Alto Alto Gállego Sabiñánigo Sardas Alto Gállego Sabiñánigo Sorripas Alto Gállego Sallent de Gállego Escarrilla Alto Gállego Sallent de Gállego Formigal Alto Gállego Sallent de Gállego Lanuza Alto Gállego Sallent de Gállego Sallent de Gállego Alto Gállego Sallent de Gállego Sandiniés Alto Gállego Yebra de Basa Yebra de Basa Bajo Cinca Ballobar Ballobar -

Premiados Porc D'or Capa Blanca 2020
PREMIO CATEGORIA EMPRESA NOMBRE DE LA EXPLOTACIÓN BRONCE 1ra cat PN Piensos Picart, S.A. Granja Can Solà Vell PLATA 1ra cat PN Coren Manuel Nogueira Vázquez ORO 1ra cat PN Ramaderia Casas SCP Can Fabré BRONCE 1ra cat TP Coren Manuel Gonzalez Álvarez PLATA 1ra cat TP INTIA S.C. Irigoyen-Rekarte J.Rekarte VI ORO 1ra cat TP INTIA Luis Albéniz Etxeverría BRONCE 1ra cat LG INTIA S.C. Irigoyen-Rekarte J.Rekarte VI PLATA 1ra cat LG INTIA Mikel Irigoien Irazoki ORO 1ra cat LG Piensos Picart, S.A. Granja Can Solà Vell BRONCE 2a cat PN Mazana Piensos Compuestos Abad Pelegay PLATA 2a cat PN Germans Ribes Baratas, SL Germans Ribes ORO 2a cat PN Cobadu Integración Matilla BRONCE 2a cat TP Coren Benito Rodríguez Vázquez PLATA 2a cat TP Cobadu Integración Matilla ORO 2a cat TP Nudesa Expocutrin BRONCE 2a cat LG La Farigoleta SCP Granja La Farigoleta PLATA 2a cat LG Ramadera Sant Andreu, S.L. Granja Bartomeus ORO 2a cat LG Roca Vallbona CB Granja Masnou BRONCE 3a cat PN Carnes Binéfar, SA Carnes Binéfar PLATA 3a cat PN Mazana Piensos Compuestos La Puebla - Agropecuaria del Isábena ORO 3a cat PN Cincaporc Minchavaques, S.L. BRONCE 3a cat TP Cooperativa Ganadera de Caspe Gallinat e Hijos, S.L. PLATA 3a cat TP SAT 322 Hermanos Chico Granja Manaderos ORO 3a cat TP Vall Companys Grup Tornos Ruiz SCP BRONCE 3a cat LG Granja Molist, SL Granja Molist-Can Toi PLATA 3a cat LG Cuarte,S.L. Briz-2 ORO 3a cat LG Pinsos Sant Antoni, S.A. -

Imágenes Contemporáneas De Un Mito Literario) José Luis Calvo Carilla Universidad De Zaragoza
archivo DE FILOLOGÍA aragonesa (afa) 69, 2013, pp. 117-140, ISSN: 0210-5624 Visiones literarias de Los Monegros (imágenes contemporáneas de un mito literario) José Luis Calvo Carilla Universidad de Zaragoza Aprendemos a llegar a cada sitio con el ánimo adecuado y, como viajeros, introduciremos sin duda un poco de todo lo que vemos y padecemos en los espacios contemplados, y éstos adquieren en gran medida el tono del carácter cambiante del paisaje: una subida pronunciada inspira pensamientos distintos que un camino llano, y las ensoñaciones del hombre son más livianas cuando sale a un claro del bosque. Nosotros somos un término de la ecuación, una nota del acorde, y sembramos la disonancia o la armonía casi a voluntad. (Robert Louis Stevenson) Resumen: La presente exposición se propone de una parte constatar la exis- tencia de un mito de contornos definidos, el del territorio aragonés conocido como Los Monegros, y delimitar su significación geográfica y sociológica; y de otra, estudiar las imágenes literarias a él asociadas. La doble significación de Los Monegros, como locus amoenus y como locus horribilis, ha generado una sucesión de recreaciones literarias a lo largo de la historia, las cuales tienen su punto de mayor intensidad en los siglos XX y XXI, con autores como Baroja, Sender, Arana y otros muchos de trayectoria más reciente. Palabras clave: Los Monegros, Aragón, paisaje, desierto, literatura, mito, locus amoenus, locus horribilis. Abstract: The present exhibition proposes on one hand to contrast the existence of a myth with defined contours: the Aragonese territory known as The Monegros and to define its sociological and geographical significance; and on the other to study the literary images that are associated to it. -

SE CANTO Impulsa Un Intercambio Sobre Empleo Inclusivo
1/10/2019 SE CANTO impulsa un intercambio sobre empleo inclusivo E-mail: [email protected] INICIO NOTICIAS DE CERCA AGENDA CON PROPIEDAD INFORMACIÓN TURÍSTICA GALERÍA TIENDA VÍDEOS Actualidad SE CANTO impulsa un intercambio sobre empleo inclusivo Incendio de un vehículo sin heridos en la A-129 También se celebró la reunión semestral de socios. El suceso ha tenido lugar entre Sariñena y Castelflorite. Fiestas de San Miguel en Sodeto y Valfarta En Valfarta, además, disfrutarán de la actuación del dance. Socios del proyecto SECANTO. La localidad francesa de Buzet sur Tarn fue el escenario del foro sobre empleo y de la reunión transfronteriza de coordinación del proyecto Interreg SE CANTO, los días 26 y 27 de septiembre. Organizado por el CBE du NET, entidad pública que promueve la inserción laboral en el Noreste Tolosain francés, el foro titulado “El empleo en Francia, ¡hablemos!” tuvo lugar el pasado jueves y contó con la participación de doce entidades públicas y privadas que intervienen en el país vecino en la aplicación de políticas de empleo. Las ponencias hicieron hincapié en la necesidad de facilitar la inclusión laboral de las personas vulnerables y en los recursos existentes en Francia encaminados hacia este fin. El presidente de la Comarca de Los Monegros, Armando Sanjuan, destacó en la apertura del foro que “conseguir la inserción social y laboral de las personas en situación de vulnerabilidad es su derecho y es nuestro deber. Ha de formar parte fundamental del auténtico medio de construcción de nuestro futuro en Europa”. Para Sanjuan, la jornada sobre el empleo es “reflejo de la apuesta de contribuir al desarrollo y mejorar la calidad de vida de nuestros habitantes que, en definitiva, es nuestro objetivo común”. -

Toponimia De La Zona Meridional De Monegros”, Luenga & Fablas, 17-18 (2013-2014), Pp
Luenga & fablas, 17-18 (2013-2014) I.S.S.N.: 1137-8328 LAX CACHO, Mª. Ángeles: “Toponimia de la zona meridional de Monegros”, Luenga & fablas, 17-18 (2013-2014), pp. 117- 144. Toponimia de la zona meridional de Monegros Mª. Ángeles LAX CACHO Resumen En este estudio de toponimia de la zona meridional de Monegros (Castejón de Monegros, La Almolda, Peñalba y Valfarta) se intenta encontrar la interpretación y la procedencia de los nombres de lugar y estudiar sus transformaciones. Consiste en la recogida y estudio lingüístico de la toponimia y apelativos de esta zona geográfica en estudio, alrededor de 900, y el intento de explicar cada uno de los topónimos registrados desde el punto de vista etimológico; de manera que el glosario de topónimos se ordena en primer lugar atendiendo a diferentes criterios: origen, evolución y características lingüísticas, y por último clasificándolos en campos semánticos. Un índice final agrupa los nombres de lugar mayor, menor y apelativos de cada municipio. Palabras clave: Monegros meridional, toponimia, estudio lexicológico. Toponymy of the southern zone of Monegros Abstract In this research about place-names of the southern zone of Monegros (Castejón de Monegros, La Almolda, Peñalba and Valfarta) we try to find their interpretation and origin in addition to the studying of their transformations. It consists in the collection and the linguistic study of the place- names and the appellatives of this geographical area under study, about nine hundred, and the attempt of explaining every registered place-name from the etymological point of view; so that the glossary of place-names of each township is sorted in the first place responding to different criteria: origin, evolution and linguistic properties; and finally classifying in semantic fields. -

Rutas En Los Monegros El Desierto Vivo De Europa
rutas en los monegros El desierto vivo de Europa ÍNDICE 02 San Caprasio en la Sierra de Alcubierre 04 Fuente Madre en Castejón de Monegros 05 Santa Quiteria en La Almolda 06 Ruta de Sijena en Villanueva de Sijena 07 Puiladrón desde Alcubierre 08 Pallaruelo 09 La Laguna en Sariñena 10 Saso de Santa Cruz en Castelflorite 11 Jubierre 12 La Gabarda 14 El Mobache y las Cías, en Marcén y Fraella 15 Recomendaciones SAN CAPRASIO EN LA SIERRA DE ALCUBIERRE RUTA: San Caprasio desde Farlete LOCALIDAD Y PUNTO DE SALIDA: FARLETE (ZARAGOZA). SANTUARIO VIRGEN DE LA SABINA. 30T0707573 UTM 4617525 / MODO DE ACCESO: SENDERISMO, BTT, TODO TERRENO. / DIFICULTAD: MEDIA / DISTANCIA APROXIMADA: 12,5 KM (SOLO IDA) / DESNIVEL: 447 / DURACIÓN DEL RECORRIDO: MEDIA JORNADA O EL DÍA COMPLETO / TIPO DE SEÑALIZACIÓN: MADERA 02 La Sierra de Alcubierre dispone de una red de pistas, que desde NOTA Se recomienda visitar el singular Santuario Virgen de la Sabina, San Caprasio conducen a diversos municipios de Los Monegros previa solicitud. Al lado del mismo se encuentra la casa del santero, que como: La Almolda, Monegrillo, Castejón de Monegros, Perdiguera actualmente se alquila como alojamiento. rutas en los monegros Más información y reservas: 976 16 30 40 y Leciñena. Por lo tanto, las rutas pueden hacerse en sentido circular o volviendo a los puntos de partida. Esta edición recoge solamente las rutas señalizadas hasta este momento. Desde la localidad de Farlete, a unos 800 metros del pueblo, nos aproximamos hasta el Santuario Virgen de la Sabina situado en lo alto. Vemos al fondo la Sierra de Alcubierre y San Caprasio que domina en la cumbre. -

Provincia De HUESC A
Provincia de HUESC A Comprende esta provincia los siguientes municipios, por partldosjudlciale s Partido de Barbastro Partido de Boltaña Abiego . Hoz de Barbastroe Abizanda . Laguarta . Adahuesca . Huerta de Vero . Aínsa. Laspuña. Alberuela de la Llena . Ilche . Albella y Jánovas . Linás de Broto . Alfántega. Laluenga . Arcusa . Mediano. Alquézar. Laperdiguera . Bárcabo . Morillo de Monclús . Azara . Lascellas . Benasque . Muro de Roda . Azlor . Mipanas . Bergua-Basarán . Olsón . Barbastro . Monzón. Bielsa . Palo. Barbuñales. Naval . Bisaurri . Plan. Berbegal . Peraltilla. Boltaña . Puértolas . Bierge. Pomar. Broto Pueyo de Araguás (El) . Buera. Ponzano . Burgasé. Rodellar . Castejón del Puente . Pozán de Vero . Campo . Sahún . Castillazuelo . Pueyo de Santa Cruz_ Castejón de Sobrarbe . San Juan de Plan . Colungo . Radiquero. Castejón de Sos . Santa María de Buil . Coscojuela de Fantova . Salas Altas. Ciamcsa . Sarsa de Surta. Costeán. Salas Bajas . Cortinas . Seira . Cregenzán . Salinas de Hoz. Coscojuela de Sobrarbe. Sesué . Fonz . Selgua . Chía . Siesta. Grado (El). Fanlo. Sin y Salinas. Fiscal . Talla . Partido de Benabarre Foradada de Tascar. Toledo de Lanata . Gerbe y Griébal . Torta . Aguinaliu . Merli . Gistain . Valle de Bardagí . Arén . Monesma de Benabarre. Guaso . Valle de Lierp. Benabarre . Montanúy. Labuerda . Villanova . Beranúy . Neril. Betesa . Olvena. Partido de Fraga Bonansa . Panillo . Bono . Perarrúa . AlbaIate de Cinca . Fraga . Cajigar. Pilzán . Alcolea de Cinca . Ontiñena . Caladrones . Puebla de Castro (La) . Ballobar . Osso. Calvera. Puebla de Fantova (La). Belver. Peñalba . Capella . Puebla de Roda (La) . Binaced . Torrente de Cinca . Caseras del Castillo . Puente de Montañana . Candasnos . Valfarta . Castanesa . Purroy de la Solana . Chalamera. Velilla de Cinca . Castigaleu. Roda de Isábena. Esplús . Zaidín. Cornudella de Baliera . Santa Liestra y San Qullez. Espés . Santoréns . Partido de Huesc a Fel . -

Anuncio De La Apertura De Cobranza
7 Julio 2020 Boletín Oficial de la Provincia de Huesca Nº 128 SELLO ADMINISTRACIÓN LOCAL DIPUTACIÓN PROVINCIAL DE HUESCA 07/07/2020 TESORERÍA RECAUDACION DE TRIBUTOS SERVICIOS CENTRALES Publicado en tablón de edictos 2335 ANUNCIO APERTURA DE COBRANZA DEL IMPUESTO SOBRE VEHÍCULOS DE TRACCIÓN MECÁNICA, IMPUESTO SOBRE BIENES INMUEBLES DE NATURALEZA URBANA Y DE CARACTERÍSTICAS ESPECIALES, AÑO 2020 Y TASAS Y PRECIOS PÚBLICOS, SEGUNDO PERÍODO DE RECAUDACIÓN AÑO 2020 . De conformidad con los artículos 23 y 24 del Reglamento General de Recaudación, aprobado por Real Decreto 939/2005, de 29 de julio, se pone en conocimiento de los contribuyentes, que desde el próximo día 15 de julio y hasta el día 19 de octubre de 2020, se con ambos inclusive, tendrá lugar la cobranza anual, por recibo, en período voluntario, del firma Sede no la IMPUESTO SOBRE VEHÍCULOS DE TRACCIÓN MECÁNICA (AÑO 2020), IMPUESTO una que en y SOBRE BIENES INMUEBLES DE NATURALEZA URBANA Y DE CARACTERÍSTICAS documento CSV menos ESPECIALES (AÑO 2020) Y DE TASAS Y PRECIOS PÚBLICOS de los conceptos, el el al períodos y ayuntamientos que se especifican, con arreglo al calendario que se publica en el con Electrónica Boletín Oficial de la Provincia y en los Edictos que se remitirán por el Servicio Provincial de obtener contiene Recaudación de Tributos Locales para su exposición en el Tablón de anuncios de los Sede acceda la respectivos Ayuntamientos. necesita de original Si originales, Ayuntamientos de los que se realiza el cobro del fuera Impuesto sobre Vehículos de Tracción validar. Mecánica (año 2020): documento firmas El realizada pudo las Electrónica. -
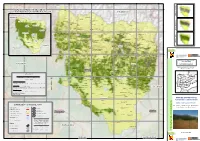
P R O V in C Ia D E H U Esc A
630 660 690 720 750 780 810 A C I F Á R G O 4750 4750 P O ANSO T MAPA DE DISTRIBUCIÓN DEL HÁBITAT D A D DE LA TRUFA NEGRA EN LA PROVINCIA DE HUESCA I FRANCIA L A I C N E T O P NAVARRA SALLENT DE GALLEGO CANFRANC BORAU PANTICOSA A C I JACETANIA VALLE DE HECHO T ARAGÜES DEL PUERTO Á M I AISA-CANDANCHU L C JASA HOZ DE JACA TORLA BIELSA D GISTAIN A SOBRARBE VILLANUA D ALTO I GÁLLEGO L A BENASQUE I C N SAN JUAN DE PLAN E BORAU T YESERO CASTIELLO DE JACA BIESCAS O SAHUN P RIBAGORZA TELLA-SIN 47 FANLO 47 20 CANAL DE BERDUN 20 HOYA DE HUESCA PUENTE LA REINA DE JACA BROTO PUERTOLAS SOMONTANO VILLANOVACASTEJON DE SOS MONTANUY A PLAN C DE Jaca SESUE I SANTA CILIA DE JACA F BARBASTRO Á D JACA CHIA E SANTA CRUZ DE LA SEROS LASPUÑA D A SEIRA D I PUEYO DE ARAGUAS BISAURRI L LA LITERA A I CINCA BAILO YEBRA DE BASA FISCAL C N MEDIO LASPAULES E T MONEGROS O LABUERDAPUEYO DE ARAGUAS P CAMPO VALLE DE BARDAJI BONANSA LLEIDA FORADADA DEL TOSCAR BOLTAÑA Aínsa VALLE DE LIERP VERACRUZ ZARAGOZA BAJO CINCA CALDEARENAS TORRE LA RIBERA LAS PEÑAS DE RIGLOS SABIÑANIGO LA FUEVA SOPEIRA AINSA-SOBRARBE 46 PALO SANTA LIESTRA Y SAN QUILEZ 46 90 ISABENA 90 ARGUIS AREN AGÜERO LOARRE NUENO PERARRUA ABIZANDA BARCABO BIERGE MONESMA Y CAJIGAR MAPA DE APTITUD PARA EL CULTIVO LA SOTONERA AYERBE CASBAS DE HUESCA DE LA TRUFA NEGRA ADAHUESCA CASTIGALEU LOSCORRALES LOPORZANO (Tuber melanosporum Vittad.) NAVAL SECASTILLA LASCUARRE NAVARRA IGRIES Graus BISCARRUES CAPELLA EN LA PROVINCIA DE HUESCA COLUNGO PUENTE DE MONTAÑANA BANASTAS ALQUEZAR CHIMILLAS HOZ Y COSTEAN Juan Barriuso ; Roberto -

2 3 1 En Quibla Mon Te Alto
UNIDAD DE MAPA 22_A-22_2 (P.K. 72+753 - P.K. 94+267) B a rr an co V a l d e C a s te j ón Los Arr eale s Las Bas uelas Sa lie na Ba jada de la Ram bla El O liv it o B a r El Ra so r a Las Co ron as n c Vald egr ame n o N -2 d 4 e 0 F u El Algar e n t La Sar da e Val d el Rey u La Paú l Mon tes Bajo s m p e Vallicet res Vald eona s Barr La Cu nar da an co d el M uert o Velillas Las T res Güe gas B ar ran co Sa so Bajo d e la Cu n a La Co ron a rda B Las Ca mp anas a rr a n c FINAL UME o d Las Er mitas e la Los Cir ios Los Valles F u Las Plan azas e An ca M ayor n Sié tamo te U m 00 50 +0 5 p 4 e 9 r Vas e ra Vel illas la Hab an an co d e Bar Barr Vel illas ran c El Sas o o Mo n del El M ur o op al s LaLass S Suerrttees s era o n La Ha ban era s arb Pa rid era d e M on to ri 0 o C n s Monte a M edias 0 Mon te a Me dias la r e 0 0 0 0 -s24 -24 0 d rNa N o o n e LaLa R Raambleetata f s 0 0 rcbo + e aan 0 o C 0 0 as rr 50 8 d l a n l e + Los Planos + d B 8 r o a n c 0 s Valdefornos 9 a Vald efor nos o rr Vf a B a 9 e 8 0 e V d 0 dl 0 Ang üés e 0 a 0 500 Pla ndele cina o d + V 0 Los Plan os c Los Yerm os 3 0 e + o n 0 Fust al c al 9 0 ad 7 c 0 s a n r 0 eñ 0 r 2 8 l P 0 a e o d 0 a 2 PPaarriizzoonnaal l San Bartolom é o + - r c c LLaa Huueerrttaa Vald etén n + A r rra B 0 Ang üés a 2 a B n T.M. -

COMUNIDAD AUTÓNOMA DE ARAGÓN Zonas Vulnerables a La
Dirección General de Calidad y COMUNIDAD AUTÓNOMA DE ARAGÓN Seguridad Alimentaria Zonas Vulnerables a la contaminación de las aguas por nitratos Orden AGM/83/2021 Ansó Ansó Sallent de Gállego Canfranc Panticosa Fago Valle de Hecho Aragüés del Puerto Aísa Jasa Hoz de Jaca Gistaín Villanúa Torla-Ordesa Bielsa Salvatierra de Esca Borau Benasque Castiello de Jaca Biescas Yésero San Juan de Plan Sahún Tella-Sin Sigüés Canal de Berdún Fanlo Artieda Puente la Reina de Jaca Broto Puértolas Undués de Lerda Mianos Plan Sesué Montanuy Santa Cilia Villanova Chía Castejón de Sos Los Pintanos Jaca Urriés Bagüés Santa Cruz de la Serós Laspuña Seira Bisaurri Laspaúles Navardún Bailo Yebra de Basa Fiscal Isuerre Labuerda Longás El Pueyo de Araguás Lobera de Onsella Sabiñánigo Valle de Bardají Sos del Rey Católico Bonansa Boltaña Foradada del Toscar Las Peñas de Riglos Caldearenas Valle de Lierp Beranuy La Fueva Sopeira Biel Aínsa-Sobrarbe Castiliscar Uncastillo Luesia Santaliestra y San Quílez Murillo de Gállego Palo Isábena Agüero Loarre Arguis Arén Nueno Layana Perarrúa El Frago Abizanda Bárcabo Monesma y Cajigar Sádaba Asín Bierge Orés Santa Eulalia de Gállego Ayerbe La Sotonera Biota Adahuesca Castigaleu Loscorrales Loporzano Graus Lascuarre Igriés Casbas de Huesca Naval Secastilla Biscarrués Capella Puente de Montañana Colungo Ardisa Chimillas Lupiñén-Ortilla Ibieca Tolva LunaValpalmas Alerre Quicena Abiego El Grado La Puebla de Castro Puendeluna Siétamo Hoz y Costeán Huesca Tierz Salas Altas Ejea de los Caballeros Piedratajada Azlor Benabarre Viacamp -

Circunscripciones Infraprovinciales
MEMORIA 2016 CIRCUNSCRIPCIONES INFRAPROVINCIALES CAPÍTULO III: CIRCUNSCRIPCIONES INFRAPROVINCIALES A. MANCOMUNIDADES DE MUNICIPIOS Existen las que se relacionan a continuación: Denominación y núcleos que las integran Finalidad 1.- ALTO VALLE DEL ARAGÓN (Aísa, Borau, Canfranc, Castiello de Jaca, Transporte Viajeros Jaca, Villanúa) estaciones Astún y Candanchú 2.- VALLE DE CHISTAU (Gistaín, Plan, San Juan de Plan, Tella-Sin) Fines varios 3.- AGUAS DE VADIELLO (Alcalá del Obispo, Argavieso, Loporzano, Sesa, Abastecimiento de Siétamo, Novales) agua 4.- AGUAS DE CALCÓN (Angüés, Casbas de Huesca, Ibieca, Loporzano) Abastecimiento de agua 5.- AGUAS DE ANTILLÓN Y 6 PUEBLOS MÁS (Angüés, Antillón, Blecua y Abastecimiento de Torres, Siétamo) agua 6.- MONEGROS II (La Almolda, Bujaraloz, Candasnos, Castejón de Abastecimiento de Monegros, Peñalba, Valfarta) agua Mancomunidades Forestales Finalidad 7.- VALLE DE BROTO (Broto, Torla-Ordesa, Linas de Broto) Aprovechamie. forestales 8.- ANSÓ-FAGO (Ansó, Fago, Industrias Forestales de Ansó, S.A.) Aprovechamie. montes 9.- ARAGÜÉS DEL PUERTO-JASA (Aragüés del Puerto y Jasa) Aprovechamie. Montes 10.- QUIÑÓN DE PANTICOSA (Hoz de Jaca, Panticosa, El Pueyo de Jaca) Aprovechamie. forestales 11.- QUIÑÓN DE BUERBA (Buerba, Nerín y Vio) Aprovechamie. Forestales 12.- LINÁS DE BROTO, BROTO Y FRAGÉN (Broto, Fragén, Linás de Broto) Aprovechamie. forestales 13.- MONTE YALI (Foradada del Toscar, Viu) Aprov. forestal de monte Yali, n.º 77 14.- SIN, SEÑÉS Y SERVETO (Serveto, Tella-Sin) Aprovechamie. Forestal 15.- VALLE DE AÍSA (Aísa, Esposa, Sinués) Aprovechamie. forestal 16.- BUESA-BROTO (Broto, Buesa) Aprov. forestal monte n.º 118 pesquera 18 MEMORIA 2016 CIRCUNSCRIPCIONES INFRAPROVINCIALES Mancomunidades de Pastos 17.- PUERTO DE ESCARRA (Escarrilla, Sandiniés y Tramacastilla de Tena) Aprovecham.