The Thermal Structure of the Anoxic Trough in Lake Untersee, Antarctica JAMES BEVINGTON1,2, CHRISTOPHER P
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Metagenomic Profiling of the Methane-Rich Anoxic Waters of Lake Untersee As an Ocean Worlds Analog
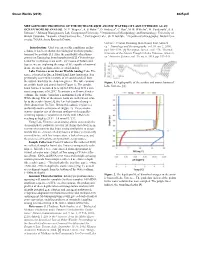
Ocean Worlds (2019) 6025.pdf METAGENOMIC PROFILING OF THE METHANE-RICH ANOXIC WATERS OF LAKE UNTERSEE AS AN OCEAN WORLDS ANALOG. N. Y. Wagner1, A. S. Hahn2,3, D. Andersen4, C. Roy5, M. B. Wilhelm6, M. Vanderwilt1, S. S. Johnson1, 1 Johnson Biosignatures Lab, Georgetown University, 2 Department of Microbiology and Immunology, University of British Columbia, 3 Koonkie Cloud Services Inc., 4 Carl Sagan Center, SETI Institute, 5 Department of Geography, McGill Uni- versity, 6NASA Ames Research Center. Untersee, Central Dronning Maud Land, East Antarcti- Introduction: Under ocean worlds conditions on En- ca.” Limnology and Oceanography, vol. 51, no. 2, 2006, celadus, it has been shown that biological methane produc- pp.1180–1194, [4] Bevington, James, et al. “The Thermal tion may be possible [1]. Also, the possibility of methano- Structure of the Anoxic Trough in Lake Untersee, Antarcti- genesis on Europa has been hypothesized [2]. Given the po- ca.”Antarctic Science, vol. 30, no. 6, 2018, pp. 333–344. tential for methanogenesis on the icy moons of Saturn and Jupiter, we are exploring the range of life capable of survival in an extremely methane-rich terrestrial analog. Lake Untersee as an Ocean Worlds Analog: Lake Un- tersee is located in Queen Maud Land, East Antarctica. It is perennially covered in 3 meters of ice and closed off from the outside world by the Anuchin glacier. The lake contains Figure 1. Depth profile of the aerobic and anoxic basins of an aerobic basin and anoxic basin (Figure 1). The aerobic Lake Untersee [4]. basin has been measured to be up to 169m deep with a con- stant temperature of 0.25˚C. -

Algal Toxic Compounds and Their Aeroterrestrial, Airborne and Other Extremophilic Producers with Attention to Soil and Plant Contamination: a Review
toxins Review Algal Toxic Compounds and Their Aeroterrestrial, Airborne and other Extremophilic Producers with Attention to Soil and Plant Contamination: A Review Georg G¨аrtner 1, Maya Stoyneva-G¨аrtner 2 and Blagoy Uzunov 2,* 1 Institut für Botanik der Universität Innsbruck, Sternwartestrasse 15, 6020 Innsbruck, Austria; [email protected] 2 Department of Botany, Faculty of Biology, Sofia University “St. Kliment Ohridski”, 8 blvd. Dragan Tsankov, 1164 Sofia, Bulgaria; mstoyneva@uni-sofia.bg * Correspondence: buzunov@uni-sofia.bg Abstract: The review summarizes the available knowledge on toxins and their producers from rather disparate algal assemblages of aeroterrestrial, airborne and other versatile extreme environments (hot springs, deserts, ice, snow, caves, etc.) and on phycotoxins as contaminants of emergent concern in soil and plants. There is a growing body of evidence that algal toxins and their producers occur in all general types of extreme habitats, and cyanobacteria/cyanoprokaryotes dominate in most of them. Altogether, 55 toxigenic algal genera (47 cyanoprokaryotes) were enlisted, and our analysis showed that besides the “standard” toxins, routinely known from different waterbodies (microcystins, nodularins, anatoxins, saxitoxins, cylindrospermopsins, BMAA, etc.), they can produce some specific toxic compounds. Whether the toxic biomolecules are related with the harsh conditions on which algae have to thrive and what is their functional role may be answered by future studies. Therefore, we outline the gaps in knowledge and provide ideas for further research, considering, from one side, Citation: G¨аrtner, G.; the health risk from phycotoxins on the background of the global warming and eutrophication and, ¨а Stoyneva-G rtner, M.; Uzunov, B. -

Extremophile Hunt Begins 8 February 2008
Extremophile Hunt Begins 8 February 2008 Hoover, "is that you don't have to have a 'Goldilocks' zone with perfect temperature, a certain pH level, and so forth, for life to thrive." Researchers have found microbes living in ice, in boiling water, in nuclear reactors. These "strange" extremophiles may in fact be the norm for life elsewhere in the cosmos. "With our research this year, we hope to identify some new limits for life in terms of temperature and pH levels. This will help us decide where to search for life on other planets and how to recognize alien life if we actually find it." Spirochaeta americana, extreme-loving microbes from California's Mono Lake. Hoover et al discovered them Hoover has already made some new friends in cold during a previous extremophile-hunting expedition. places. Earlier Hoover teams have found new species and genera of anaerobic microbial extremophiles in the ice and permafrost of Alaska, Siberia, Patagonia, and Antarctica. A team of scientists has just left the country to explore a very strange lake in Antarctica; it is filled "I found one extremophile in penguin guano," with, essentially, extra-strength laundry detergent. recalls Hoover. "When I stooped to pick it up, Jim No, the researchers haven't spilled coffee on their Lovell, my research partner then, said, 'What the lab coats. They are hunting for extremophiles -- heck are you doing now, Richard?' But it paid off." tough little creatures that thrive in conditions too extreme for most other living things. Most incredible, though, was the revelation a few years ago that some extremophiles the researchers Antarctica's Lake Untersee, fed by glaciers, always found in an Alaskan tunnel actually came to life as covered with ice, and very alkaline, is one of the the ice around them melted. -

Antarctic Research As a Precedent for Diplomacy Exercised Through International Scientific Cooperation: Applying Collaborative Systems in Non-Jurisdictional Spaces
Antarctic research as a precedent for diplomacy exercised through international scientific cooperation: applying collaborative systems in non-jurisdictional spaces Mia Vanderwilt, B.S. Science, Technology and International Affairs, Environment & Energy Senior Thesis Accompaniment Acknowledgements This paper accompanies a senior thesis manuscript on the effect of heightened soil moisture and salinity on soil microbial communities within an Antarctic water track. Field work for this thesis was conducted as a part of the 2018 Tawani Expedition to Lake Untersee, Antarctica, a hyperarid Mars-analog environment. This supplementary paper will examine how multinational Antarctic research exemplifies the potential of scientific cooperation to mediate diplomacy in non- jurisdictional areas. Specifically, this paper will briefly examine how multilateral Antarctic treaties prioritizing collaborative research have facilitated scientific diplomacy, minimized geopolitical conflict and mitigated threats of militarization. This paper will be submitted along with the manuscript for honors to the Science, Technology, and International Affairs program within the School of Foreign Service at Georgetown University. Introduction A significant percentage of the Earth’s surface is non-jurisdictional, falling outside traditional bounds of national sovereignty. The vast majority of this area lies within polar regions and the high oceans beyond the reach of national exclusive economic zones. Successes and failures in the governance of these spaces in the absence of sovereign authorities provide valuable lessons for the avoidance of further militarization and destructive resource extraction in continued polar, ocean and space exploration. Antarctica serves as an ideal case study for how initial militarization and territorial contests were successfully diffused through the enactment of an international treaty formally prioritizing international scientific research. -

Curriculum Vitae Dale T. Andersen Email
Curriculum Vitae Dale T. Andersen Email: [email protected] Current Position: Senior Research Scientist, Carl Sagan Center for the Study of Life in the Universe, SETI Institute. 189 Bernardo Ave. Suite 200, Mountain View, CA 94043. Academic Degrees: PhD, McGill University, 2004. Physical Geography. BS, Va Tech, 1979. Biology. Professional Background: 1993-present, SETI Institute. Experienced limnologist/aquatic ecologist with a long history of work in polar-regions and temperate deserts (Mojave, Atacama). Developed techniques for scientific diving and the use of ROV technology for the exploration of perennially ice-covered lakes in Antarctica; led the first comprehensive studies of perennial spring ecosystems of Axel Heiberg in the High Arctic; led the first expedition to explore the sub- ice environment of Lakes Untersee and Obersee in the mountains of Queen Maud Land, Antarctica using technical diving, discovering the only know modern large conical stromatolites. Extensive experience with EPO having created two PBS documentaries including the only live interactive broadcast from beneath Antarctic sea-ice with participation of middle schools in the US including early online blogs (1993); Polar, Research related images & video used by National Geographic, PBS, Discovery Channel, NASA, Nikon, and others for various magazines, journals and television programming, as well as non-fiction books. Contributed essay titled: Life Under the Arctic Ice." National Geographic Ocean: An Illustrated Atlas (2008); as a member of the NASA Exobiology Implementation Team within the US/USSR Joint Working Group for Space Medicine and Biology, led the US team during a joint US/Soviet expedition to the Bunger Hills, Antarctica to study perennially ice-covered lakes in that oasis region. -

Walled Lake Untersee, Antarctica
LIMNOLOGY and Limnol. Oceanogr. 00, 2015, 00–00 OCEANOGRAPHY VC 2015 Association for the Sciences of Limnology and Oceanography doi: 10.1002/lno.10086 Modeling circulation and seasonal fluctuations in perennially ice-covered and ice-walled Lake Untersee, Antarctica H. C. B. Steel,1 C. P. McKay,1 D. T. Andersen*2 1MS-245-3 NASA Ames Research Center Moffett Field, California 2Carl Sagan Center, SETI Institute, Mountain View, California Lake Untersee, Antarctica, is a freshwater perennially ice covered lake bounded along its north by the Anu- chin glacier. The Massachusetts Institute of Technology general circulation model, used on a representative wedge-shaped lake and actual bathymetry for Lake Untersee, produces estimates for circulation and long- term temperature and mixing trends. Modeled circulation is dominated by an anticyclonic gyre in front of the glacier, with slower 3mms21 flow exhibited around the lake’s perimeter, allowing effective mixing throughout most of the lake with time scales of one month. Estimated velocities bound maximal glacial flour particle size at 10 lm for effective transport throughout the lake, consistent with the sediment’s mostly fine composition observed in field studies, and mixing time scales mean nonuniformities in measured O2 concentration likely require recent or ongoing sources. Areas in which large temperature gradients prevent exchange of fluid demonstrate minimal mixing, such as the lake’s upper water layers and the anoxic basin in the south, and circulation is consistently slowed in the northern sheltered bay area. Mean flow velocities fluctuate by about one fifth of their magnitude between summer and winter, and the lake’s almost homo- thermal body temperature varies by about one tenth of a degree over the same period. -

PSYCHROPHILIC and PSYCHROTOLERANT MICROBIAL EXTREMOPHILES in POLAR ENVIRONMENTS Richard B
https://ntrs.nasa.gov/search.jsp?R=20100002095 2019-08-30T08:31:58+00:00Z 1 PSYCHROPHILIC AND PSYCHROTOLERANT MICROBIAL EXTREMOPHILES IN POLAR ENVIRONMENTS Richard B. Hoovera and Elena V. Pikutab aSpace Science Office, Mail Code 62, NASA/Marshall Space Flight Center, Huntsville, AL 35812 bNational Space Science and Technology Center, 320 Sparkman Dr., Huntsville, AL 35805, USA [email protected] CONTENTS 1. INTRODUCTION 2. PRODUCERS OF ORGANIC MATTER IN POLAR ENVIRONMENTS 2.1 Eukaryotic Photosynthetic Microorganisms. 2.1.1 Diatoms 2.1.2 Snow Algae 2.1.3 Prokaryotic Photosynthetic Microorganisms 2.1.4 Bioremediation by Diatoms and Cyanobacteria 2.2. Psychrophilic and Psychrotolerant Anaerobic Chemolithotrophic Autotrophs 2.2.1 Methanogens 2.2.2 Acetogens 3. DECOMPOSERS OF ORGANIC MATTER IN POLAR ENVIRONMENTS 4. EXTREMOPHILES WITHIN PHYSICO-CHEMICAL MATRIX 5. ANAEROBIC EXTREMOPHILES FROM POLAR EXPEDITIONS 5.1 Novel Psychrotolerant Extremophiles from Expeditions to Alaska 5.1.1 Pleistocene Bacterium from Fox Permafrost Tunnel 5.1.2 Novel Acidophile from Chena Hot Springs 5.2 Novel Extremophiles from Antarctica 2000 Expedition 5.2.1 Psychrotolerant Anaerobes from Magellanic Penguin Colony in Southern Patagonia, Chile 5.2.2 Novel Psychrophilic and Psychrotolerant Anaerobes from Patriot Hills, Antarctica 5.3 2008 Tawani International Antarctica Expeditions 5.3.1 Psychrotolerant Anaerobes from the African Penguin guano 5.3.2 Microbial Extremophiles from the Schirmacher Oasis, Antarctica 5.3.2.1 Bacteria from Lake Zub (Lake Priyadarshini) 5.3.2.2 Bacteria from Ice Sculptures near Lake Podprudnoye 5.4 Microbial Extremophiles from Lake Untersee 5.4.1 Psychrophilic and Psychrotolerant Anaerobes from Lake Untersee 5.5 Microorganisms in-situ in Ice-Bubbles 6.0 RELEVANCE OF POLAR MICROBIAL EXTREMOPHILES TO ASTROBIOLOGY 7.0 SUMMARY 8.0 REFERENCES Acknowledgements 1 2 1. -

Microbial Cooking, Cleaning, and Control Under Stress
life Review Housekeeping in the Hydrosphere: Microbial Cooking, Cleaning, and Control under Stress Bopaiah Biddanda 1,* , Deborah Dila 2 , Anthony Weinke 1 , Jasmine Mancuso 1 , Manuel Villar-Argaiz 3 , Juan Manuel Medina-Sánchez 3 , Juan Manuel González-Olalla 4 and Presentación Carrillo 4 1 Annis Water Resources Institute, Grand Valley State University, Muskegon, MI 49441, USA; [email protected] (A.W.); [email protected] (J.M.) 2 School of Freshwater Sciences, University of Wisconsin-Milwaukee, Milwaukee, WI 53204, USA; [email protected] 3 Departamento de Ecología, Facultad de Ciencias, Universidad de Granada, 18071 Granada, Spain; [email protected] (M.V.-A.); [email protected] (J.M.M.-S.) 4 Instituto Universitario de Investigación del Agua, Universidad de Granada, 18071 Granada, Spain; [email protected] (J.M.G.-O.); [email protected] (P.C.) * Correspondence: [email protected]; Tel.: +1-616-331-3978 Abstract: Who’s cooking, who’s cleaning, and who’s got the remote control within the waters blan- keting Earth? Anatomically tiny, numerically dominant microbes are the crucial “homemakers” of the watery household. Phytoplankton’s culinary abilities enable them to create food by absorbing sunlight to fix carbon and release oxygen, making microbial autotrophs top-chefs in the aquatic kitchen. However, they are not the only bioengineers that balance this complex household. Ubiqui- tous heterotrophic microbes including prokaryotic bacteria and archaea (both “bacteria” henceforth), Citation: Biddanda, B.; Dila, D.; eukaryotic protists, and viruses, recycle organic matter and make inorganic nutrients available to Weinke, A.; Mancuso, J.; Villar-Argaiz, primary producers. Grazing protists compete with viruses for bacterial biomass, whereas mixotrophic M.; Medina-Sánchez, J.M.; protists produce new organic matter as well as consume microbial biomass. -

Lichens, Bryophytes and Terrestrial Algae of the Lake Untersee Oasis (Wohlthat Massiv, Dronning Maud Land, Antarctica)
CZECH POLAR REPORTS 10 (2): 203-225, 2020 Lichens, bryophytes and terrestrial algae of the Lake Untersee Oasis (Wohlthat Massiv, Dronning Maud Land, Antarctica) Mikhail Andreev1*, Dale Andersen2, Lyubov Kurbatova1, Svetlana Smirnova1, Olga Chaplygina1 1Komarov Botanical Institute of the Russian Academy of Sciences, Professor Popov St. 2, 197376 St. Petersburg, Russia 2Carl Sagan Center, SETI Institute, 189 Bernardo Ave., Suite 100, Mountain View, California 94043, USA Abstract Lake Untersee is the largest ice-covered freshwater lake in the interior of East Antarctica. The mountain oasis is situated around it in the Gruber Mts. of the Wohlthat Massif. For approximately 7,000 years the area has been free of ice and the local climate relatively stable. It is very severe, cold, and windy and dominated by intense evaporation and sublimation but with little melt. Relative humidity averages only 37%. Vegetation is sparse in the oasis and previously only poorly investigated. Two lichen species and no bryophytes were known from the area. In November-December 2018, a survey of terrestrial flora and vegetation was made. The list of lichens was completed for the area, bryophytes were found for the first time, and some terrestrial algae were collected. In total, 23 lichen species, 1 lichenicolous fungus, 1 moss, and 18 terrestrial algae were discovered for the locality. The abundance of each species within their habitats was also evaluated. The lichen flora of the Untersee Oasis is typical for continental oases and similar to other previously investigated internal territories of Dronning Maud Land, except for the very rich lichen flora of the Schirmacher Oasis. -

Search for a Second Genesis of Life on Other Worlds in the Solar System
Search for a second genesis of life on other worlds in the Solar System 24 Oct 2016 CCST [email protected] The search for a second genesis of life ⇒ comparave biochemistry (life 2.0) ⇒ life is common in the universe (yeah!) (by the zero-one-infinity rule) Aliens: not on our tree of life "The Tree of Life" defines Earth Life 2 Second Genesis:! ! How will we get our second example of Biochemistry! Listen for them to call ! ! Make it in the laboratory! Find it on another world! Where to look for life? Mars ★★ Europa x (moon of Jupiter) Enceladus (moon of Saturn) Titan ?! (moon of Saturn) Curiosity on Mars Landed 5 August 2012 Yellowknife Bay, Mars Yellowknife Bay: An ideal site for astrobiology •" 3500 Myr ago; Impact forms Gale Crater •" Soon thereafter water deposits sediments. They harden and become compact and are buried •" Exposed 70 Myr ago by wind erosion of upper later Lake Untersee, East Antarctica On the bottom of an ice-covered lake. A world of only microscopic life making large mounds. Analogs for early Earth and Mars. N2, O2, Ar Tmax>0ºC, P>Pt Dry Valley Lakes Glacier Summer melt Ice cover N2/Ar in melt ~ ½ air value Tmax<0ºC, P<Pt Lake Untersee N , O , Ar 2 2 Ice cover Glacier Subaqueus melting N2/Ar in melt ~ air value Curiosity Big Result #1 : Grey Mars Zagami O2 and H2S and a small amount of organics New drill hole and even darker material beneath. New Possible Ocean on Europa Europa: Reaching the Ocean Jets of H2O on Enceladus LIFE Life Investigation For Enceladus P. -

Hydrochemistry of Ice-Covered Lakes and Ponds in the Untersee Oasis (Queen Maud Land, Antarctica)
Hydrochemistry of ice-covered lakes and ponds in the Untersee Oasis (Queen Maud Land, Antarctica) Benoit Faucher Thesis submitted to the University of Ottawa in partial Fulfillment of the requirements for the Doctorate of Philosophy in Geography Department of Geography, Environment, and Geomatics Faculty of Arts University of Ottawa © Benoit Faucher, Ottawa, Canada, 2021 ABSTRACT Several thousand coastal perennially ice-covered oligotrophic lakes and ponds have been identified on the Antarctic continent. To date, most hydrochemical studies on Antarctica’s ice- covered lakes have been undertaken in the McMurdo Dry Valleys (more than 20 lakes/ponds studied since 1957) because of their proximity to the McMurdo research station and the New Zealand station Scott Base. Yet, little attention has been given to coastal ice-covered lakes situated in Antarctica’s central Queen Maud Land region, and more specifically in the Untersee Oasis: a polar Oasis that encompasses two large perennially ice-covered lakes (Lake Untersee & Lake Obersee), and numerous small ice-covered morainic ponds. Consequently, this PhD research project aims to describe and understand the distribution, ice cover phenology, and contemporary hydrochemistry of perennially ice-covered lakes and ponds located in the Untersee Oasis and their effect on the activity of the benthic microbial ecosystem. Lake Untersee, the largest freshwater coastal lake in central Queen Maud Land, was the main focus of this study. Its energy and water mass balance was initially investigated to understand its current equilibrium and how this perennially well-sealed ice-covered lake may evolve under changing climate conditions. Results suggest that Lake Untersee’s mass balance was in equilibrium between the late 1990s and 2018, and the lake is mainly fed by subglacial meltwater (55-60%) and by subaqueous melting of glacier ice (40-45%). -

Carbon-Cycling in Lake Untersee, Dronning Maud Land, East Antarctica
Goldschmidt2018 Abstract Carbon-Cycling in Lake Untersee, Dronning Maud Land, East Antarctica N.B. MARSH1*, D. LACELLE1, I.D. CLARK1, B. FAUCHER1, D.T. ANDERSEN2 1University of Ottawa, Ottawa, Ontario, Canada (*correspondence: [email protected]) 2SETI Institute, Mountain View, California, USA ([email protected]) Lake Untersee is one of the largest (11.3 km2) and deepest (>160 m) freshwater lakes in East Antarctica. Perennially ice- covered and bounded at one end by the Anuchin Glacier, the lake hydrological balance is controled by input from englacial meltwater and output by sublimation of the ice-cover [1]. The lake is well-mixed, alkaline (pH ~ 10), and supersaturated with dissolved oxygen (~ 150%), with the exception of an anoxic basin in the southwest of the lake that is dominated by methanogenesis processes [1,2]. The lake supports exclusively a microbial ecosystem with no higher plants, invertebrates, or fish. The lake water column is clear and ultra-oligotrophic, with volumetric planktonic primary productivity close to the lowest on record. Meltwater from the glacier contribute solutes and gases to the lake, whereas microbial communities populating the bottom of the lake may influence the carbon isotope signature 13 14 ( C- and C-CO2) through carbon fixation (i.e., photosynthesis) by cyanobacteria and/or and CO2 respiration by heterotrophs. The objective is to investigate geochemical evolution and trace carbon cycling and weathering in Lake Untersee through major and trace element geochemistry and compound-specific 13 carbon isotopic analysis. Results for DIC, DOC, and δ CDIC show stable values through the oxic water column (0.3-0.4ppm, 0-0.2ppm and -10 to -7‰, respectively).