Microbial Cooking, Cleaning, and Control Under Stress
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Shedding Light on Microbial Dark Matter with a Universal Language
bioRxiv preprint doi: https://doi.org/10.1101/2020.12.23.424215; this version posted December 26, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. 1 Shedding Light on Microbial Dark Matter with A 2 Universal Language of Life 1,* 2 3 2,* 3 Hoarfrost A , Aptekmann, A , Farfañuk, G , Bromberg Y 4 1 Department of Marine and Coastal Sciences, Rutgers University, 71 Dudley Road, New Brunswick, NJ 08873, USA 5 2 Department of Biochemistry and Microbiology, 76 Lipman Dr, Rutgers University, New Brunswick, NJ 08901, USA 6 3 Department of Biological Chemistry, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Argentina 7 8 * Corresponding author: [email protected]; [email protected] 9 Running Title: LookingGlass: a universal language of life 10 11 Abstract 12 The majority of microbial genomes have yet to be cultured, and most proteins 13 predicted from microbial genomes or sequenced from the environment cannot be 14 functionally annotated. As a result, current computational approaches to describe 15 microbial systems rely on incomplete reference databases that cannot adequately 16 capture the full functional diversity of the microbial tree of life, limiting our ability to 17 model high-level features of biological sequences. The scientific community needs a 18 means to capture the functionally and evolutionarily relevant features underlying 19 biology, independent of our incomplete reference databases. Such a model can form 20 the basis for transfer learning tasks, enabling downstream applications in 21 environmental microbiology, medicine, and bioengineering. -

Aquatic Ecosystems
February 19, 2014 Nantahala and Pisgah NFs Assessment Aquatic Ecosystems The overall richness of North Carolina’s aquatic fauna is directly related to the geomorphology of the state, which defines the major drainage divisions and the diversity of habitats found within. There are seventeen major river basins in North Carolina. Five western basins are part of the Interior Basin (IB) and drain to the Mississippi River and the Gulf of Mexico (Hiwassee, Little Tennessee, French Broad, Watauga, and New). Parts of these five river basins are within the Nantahala and Pisgah National Forests (NFs). Twelve central and eastern basins are part of the Atlantic Slope (AS) and flow to the Atlantic Ocean. Of these twelve central and eastern basins, parts of the Savannah, Broad, Catawba, and Yadkin-Pee Dee basins are within the Nantahala and Pisgah NFs. As described later in this report, the Nantahala and Pisgah NFs, for the most part, support higher elevation coldwater streams, and relatively little cool- and warmwater resources. To gain perspective on the importance of aquatic ecosystems on the Nantahala and Pisgah NFs, it is first necessary to understand their value at regional and national scales. The southeastern United States has the highest aquatic species diversity in the entire United States (Burr and Mayden 1992; Williams et al. 1993; Taylor et al. 1996; Warren et al. 2000,), with southeastern fishes comprising 62% of the United States fauna, and nearly 50% of the North American fish fauna (Burr and Mayden 1992). Freshwater mollusk diversity in the southeast is ‘globally unparalleled’, representing 91% of all United States mussel species (Neves et al. -
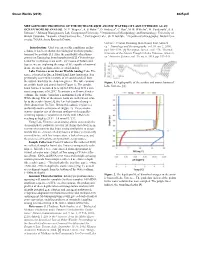
Metagenomic Profiling of the Methane-Rich Anoxic Waters of Lake Untersee As an Ocean Worlds Analog
Ocean Worlds (2019) 6025.pdf METAGENOMIC PROFILING OF THE METHANE-RICH ANOXIC WATERS OF LAKE UNTERSEE AS AN OCEAN WORLDS ANALOG. N. Y. Wagner1, A. S. Hahn2,3, D. Andersen4, C. Roy5, M. B. Wilhelm6, M. Vanderwilt1, S. S. Johnson1, 1 Johnson Biosignatures Lab, Georgetown University, 2 Department of Microbiology and Immunology, University of British Columbia, 3 Koonkie Cloud Services Inc., 4 Carl Sagan Center, SETI Institute, 5 Department of Geography, McGill Uni- versity, 6NASA Ames Research Center. Untersee, Central Dronning Maud Land, East Antarcti- Introduction: Under ocean worlds conditions on En- ca.” Limnology and Oceanography, vol. 51, no. 2, 2006, celadus, it has been shown that biological methane produc- pp.1180–1194, [4] Bevington, James, et al. “The Thermal tion may be possible [1]. Also, the possibility of methano- Structure of the Anoxic Trough in Lake Untersee, Antarcti- genesis on Europa has been hypothesized [2]. Given the po- ca.”Antarctic Science, vol. 30, no. 6, 2018, pp. 333–344. tential for methanogenesis on the icy moons of Saturn and Jupiter, we are exploring the range of life capable of survival in an extremely methane-rich terrestrial analog. Lake Untersee as an Ocean Worlds Analog: Lake Un- tersee is located in Queen Maud Land, East Antarctica. It is perennially covered in 3 meters of ice and closed off from the outside world by the Anuchin glacier. The lake contains Figure 1. Depth profile of the aerobic and anoxic basins of an aerobic basin and anoxic basin (Figure 1). The aerobic Lake Untersee [4]. basin has been measured to be up to 169m deep with a con- stant temperature of 0.25˚C. -

Aquatic Ecology ______INTRODUCTION
Aquatic Ecology ________________________________________________________________ INTRODUCTION Aquatic Ecology Field Station Aquatics or aquatic ecology is the study of animals and plants in freshwater environments. In addition to the many common aquatic species in this Western New York region, a student of aquatics learns about watersheds, wetlands and the hydrologic cycle. Essential to understanding and appreciating the field of aquatics is a basic knowledge of the physical and chemical properties of water. Water is arguably the most valuable substance on the planet, and is the common name applied to the liquid state of the hydrogen oxygen compound H2O. It covers 70% of the surface of the Earth forming swamps, lakes, rivers, and oceans. Pure water has a blue tint, which may be detected only in layers of considerable depth. It has no taste or odor. Water molecules are strongly attracted to one another through their two hydrogen atoms. At the surface, this attraction produces a tight film over the water (surface tension). A number of organisms live both on the upper and lower sides of this film. 1 Density of water is greatest at 39.2° Fahrenheit (4° Celsius). It becomes less as water warms and, more important, as it cools to freezing at 32° Fahrenheit (0° Celsius), and becomes ice. Ice is a poor heat conductor. Therefore, ice sheets on ponds, lakes and rivers trap heat in the water below. For this reason, only very shallow water bodies never freeze solid. Water is the only substance that occurs at ordinary temperatures in all three states of matter: solid, liquid, and gas. In its solid state, water is ice, and can be found as glaciers, snow, hail, and frost and ice crystals in clouds. -

Actinobacterial Rare Biospheres and Dark Matter Revealed in Habitats of the Chilean Atacama Desert
Idris H, Goodfellow M, Sanderson R, Asenjo JA, Bull AT. Actinobacterial Rare Biospheres and Dark Matter Revealed in Habitats of the Chilean Atacama Desert. Scientific Reports 2017, 7(1), 8373. Copyright: © The Author(s) 2017. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. DOI link to article: https://doi.org/10.1038/s41598-017-08937-4 Date deposited: 18/10/2017 This work is licensed under a Creative Commons Attribution 4.0 International License Newcastle University ePrints - eprint.ncl.ac.uk www.nature.com/scientificreports OPEN Actinobacterial Rare Biospheres and Dark Matter Revealed in Habitats of the Chilean Atacama Received: 13 April 2017 Accepted: 4 July 2017 Desert Published: xx xx xxxx Hamidah Idris1, Michael Goodfellow1, Roy Sanderson1, Juan A. Asenjo2 & Alan T. Bull3 The Atacama Desert is the most extreme non-polar biome on Earth, the core region of which is considered to represent the dry limit for life and to be an analogue for Martian soils. -

UC Berkeley Electronic Theses and Dissertations
UC Berkeley UC Berkeley Electronic Theses and Dissertations Title Interactions of Microbes in Communities Permalink https://escholarship.org/uc/item/2hg5h7k6 Author Sczesnak, Andrew Publication Date 2018 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California Interactions of Microbes in Communities By Andrew Sczesnak A dissertation submitted in partial satisfaction of the requirements for the degree of Joint Doctor of Philosophy with University of California, San Francisco in Bioengineering in the Graduate Division of the University of California, Berkeley Committee in charge: Professor Adam P. Arkin, Chair Professor Matthew Traxler Professor Michael Fischbach Fall 2018 Abstract Interactions of Microbes in Communities By Andrew Sczesnak Joint Doctor of Philosophy with University of California, San Francisco in Bioengineering University of California, Berkeley Professor Adam P. Arkin, Chair Groups of microorganisms sharing an environment (microbial communities) are ubiquitous in nature. Microbial communities provide essential ecosystem services to other life on Earth by e.g., participating in global biogeochemical processes or interacting with a host’s immune system. Such microbes compete for scarce resources, modify an environment for their own purposes, actively war, and occasionally cooperate. Though numerous studies have surveyed the diversity of microbial life in different environments, few have determined the ways in which members of microbial communities interact with one another. Understanding the ways and means by which microbes interact is essential if we are to understand how microbial communities form, persist, and change over time. Knowledge of these processes will allow us to rationally design microbial communities to perform useful functions and predict how our actions might shift the balance of microbes in a community, and thus affect its function. -

Algal Toxic Compounds and Their Aeroterrestrial, Airborne and Other Extremophilic Producers with Attention to Soil and Plant Contamination: a Review
toxins Review Algal Toxic Compounds and Their Aeroterrestrial, Airborne and other Extremophilic Producers with Attention to Soil and Plant Contamination: A Review Georg G¨аrtner 1, Maya Stoyneva-G¨аrtner 2 and Blagoy Uzunov 2,* 1 Institut für Botanik der Universität Innsbruck, Sternwartestrasse 15, 6020 Innsbruck, Austria; [email protected] 2 Department of Botany, Faculty of Biology, Sofia University “St. Kliment Ohridski”, 8 blvd. Dragan Tsankov, 1164 Sofia, Bulgaria; mstoyneva@uni-sofia.bg * Correspondence: buzunov@uni-sofia.bg Abstract: The review summarizes the available knowledge on toxins and their producers from rather disparate algal assemblages of aeroterrestrial, airborne and other versatile extreme environments (hot springs, deserts, ice, snow, caves, etc.) and on phycotoxins as contaminants of emergent concern in soil and plants. There is a growing body of evidence that algal toxins and their producers occur in all general types of extreme habitats, and cyanobacteria/cyanoprokaryotes dominate in most of them. Altogether, 55 toxigenic algal genera (47 cyanoprokaryotes) were enlisted, and our analysis showed that besides the “standard” toxins, routinely known from different waterbodies (microcystins, nodularins, anatoxins, saxitoxins, cylindrospermopsins, BMAA, etc.), they can produce some specific toxic compounds. Whether the toxic biomolecules are related with the harsh conditions on which algae have to thrive and what is their functional role may be answered by future studies. Therefore, we outline the gaps in knowledge and provide ideas for further research, considering, from one side, Citation: G¨аrtner, G.; the health risk from phycotoxins on the background of the global warming and eutrophication and, ¨а Stoyneva-G rtner, M.; Uzunov, B. -

Aquatic Ecosystem Delineation and Description Guidelines AQUATIC ECOSYSTEMS TOOLKIT • MODULE 4 •Aquatic Ecosystem Delineation and Description Guidelines
Aquatic ecosystems toolkit MODULE 4: Aquatic ecosystem delineation and description guidelines AQUATIC ECOSYSTEMS TOOLKIT • MODULE 4 •Aquatic ecosystem delineation and description guidelines Published by Department of Sustainability, Environment, Water, Population and Communities Authors/endorsement Aquatic Ecosystems Task Group Endorsed by the Standing Council on Environment and Water, 2012. © Commonwealth of Australia 2012 This work is copyright. You may download, display, print and reproduce this material in unaltered form only (retaining this notice) for your personal, non-commercial use or use within your organisation. Apart from any use as permitted under the Copyright Act 1968 (Cwlth), all other rights are reserved. Requests and enquiries concerning reproduction and rights should be addressed to Department of Sustainability, Environment, Water, Population and Communities, Public Affairs, GPO Box 787 Canberra ACT 2601 or email <[email protected]>. Disclaimer The views and opinions expressed in this publication are those of the authors and do not necessarily reflect those of the Australian Government or the Minister for Sustainability, Environment, Water, Population and Communities. While reasonable efforts have been made to ensure that the contents of this publication are factually correct, the Commonwealth does not accept responsibility for the accuracy or completeness of the contents, and shall not be liable for any loss or damage that may be occasioned directly or indirectly through the use of, or reliance on, the -

Isolation and Identification of Denitrifying Bacteria and Its Growth and Metabolism Characteristics
2017 2nd International Conference on Environmental Science and Energy Engineering (ICESEE 2017) ISBN: 978-1-60595-417-2 Isolation and Identification of Denitrifying Bacteria and Its Growth and Metabolism Characteristics Xin-ran JIANG, Dian JIAO, Lei ZHANG and Li-na ZHENG College of Marine Technology and Environmental, Dalian Ocean University, Dalian 116023, China Keywords: Denitrification, Nitrate, Isolation and identification, Activity research. Abstract. A denitrifying bacterium DN20 was isolated from the biofilm after centrifugation in the aquaculture purification system of a aquaculture base, and the denitrification characteristics of the strain were further studied. The strain was identified by 16S rDNA sequence analysis, the effects of temperature, salinity, pH, substrate concentration and C/N ratio on denitrification activity were studied. Introduction Denitrifying bacteria are a kind of bacteria that can cause denitrification. Biological denitrification is the use of denitrifying bacteria, nitrate nitrogen into gaseous products to remove, is considered to be the most economical and effective way of nitrogen removal [1]-[2]. The application of denitrifying bacteria and the removal of nitrate in water have become the research focus[3]-[5], many methods have been reported [6]-[8]. In recent years, some studies on salt tolerant and halophilic denitrifying bacteria have been reported abroad [9]-[10]. In this study, the sludge from the culture pond was taken as the main separation source, and the enrichment, separation and purification were carried out.To obtain a highly efficient nitrate removal ability of denitrifying bacteria, named strain DN20. The aim of this study is to provide effective bacteria source for practical application of nitrate-N treatment in aquaculture water. -

Aquatic Ecosystems Bibliography Compiled by Robert C. Worrest
Aquatic Ecosystems Bibliography Compiled by Robert C. Worrest Abboudi, M., Jeffrey, W. H., Ghiglione, J. F., Pujo-Pay, M., Oriol, L., Sempéré, R., . Joux, F. (2008). Effects of photochemical transformations of dissolved organic matter on bacterial metabolism and diversity in three contrasting coastal sites in the northwestern Mediterranean Sea during summer. Microbial Ecology, 55(2), 344-357. Abboudi, M., Surget, S. M., Rontani, J. F., Sempéré, R., & Joux, F. (2008). Physiological alteration of the marine bacterium Vibrio angustum S14 exposed to simulated sunlight during growth. Current Microbiology, 57(5), 412-417. doi: 10.1007/s00284-008-9214-9 Abernathy, J. W., Xu, P., Xu, D. H., Kucuktas, H., Klesius, P., Arias, C., & Liu, Z. (2007). Generation and analysis of expressed sequence tags from the ciliate protozoan parasite Ichthyophthirius multifiliis BMC Genomics, 8, 176. Abseck, S., Andrady, A. L., Arnold, F., Björn, L. O., Bomman, J. F., Calamari, D., . Zepp, R. G. (1998). Environmental effects of ozone depletion: 1998 assessment. Journal of Photochemistry and Photobiology B: Biology, 46(1-3), 1-108. doi: Doi: 10.1016/s1011-1344(98)00195-x Adachi, K., Kato, K., Wakamatsu, K., Ito, S., Ishimaru, K., Hirata, T., . Kumai, H. (2005). The histological analysis, colorimetric evaluation, and chemical quantification of melanin content in 'suntanned' fish. Pigment Cell Research, 18, 465-468. Adams, M. J., Hossaek, B. R., Knapp, R. A., Corn, P. S., Diamond, S. A., Trenham, P. C., & Fagre, D. B. (2005). Distribution Patterns of Lentic-Breeding Amphibians in Relation to Ultraviolet Radiation Exposure in Western North America. Ecosystems, 8(5), 488-500. Adams, N. -

Aquatic Ecosystem Part 1 a SHORT NOTE for B.SC ZOOLOGY
2020 Aquatic ecosystem part 1 A SHORT NOTE FOR B.SC ZOOLOGY WRITTEN BY DR.MOTI LAL GUPTA ,H.O.D ,DEPARTMENT OF ZOOLOGY,B.N.COLLEGE,PATNA UNIVERSITY [Type the author name] DEPARTMENT OF ZOOLOGY,B.N COLLEGE, P.U 4/16/2020 Aquatic ecosystem part 1 2 1 Department of zoology,B.N College,P.U Page 2 Aquatic ecosystem part 1 3 Contents 1. Learning Objectives 2. Introduction 3. The Lentic Aquatic System Zonation in Lentic Systems Characteristics of Lentic Ecosystem Lentic Community Communities of the littoral zone Communities of Limnetic Zone Communities of Profundal Zone 4. Lake Ecosystem Thermal Properties of Lake Seasonal Cycle in Temperate Lakes Biological Oxygen Demand Eutrophy and Oligotrophy Langmuir Circulation and the Descent of the Thermocline Types of Lakes 5. State of Freshwater Ecosystems in Present Scenario Causes of Change in the properties of freshwater bodies Climate Change Change in Water Flow Land-Use Change Changing Chemical Inputs Aquatic Invasive Species Harvest Impact of Change on Freshwater Bodies Physical Transformations 6. Summary 2 Department of zoology,B.N College,P.U Page 3 Aquatic ecosystem part 1 4 1. Learning Objectives After the end of this module you will be able to 1. Understand the concept of fresh water ecosystem. 2. Understand the characteristics of the Lentic ecosystems. 3. Know the communities of lentic ecosystems and their ecological adaptations. 4. Know properties of Lake Ecosystems and their types. 5. Understand the major changes that are causing the threats to freshwaters ecosystems. 2. Introduction Freshwater ecology can be interpreted as interrelationship between freshwater organism and their natural environments. -

Bacteria and Cyanobacteria
Bacteria and Cyanobacteria Bacteria represent an amazingly diverse group of organisms that are of great ecological, economic, agricultural, and medical importance. Cyanobacteria, also known as blue-green bacteria, contain thylakoid membranes with photosynthetic pigments (chlorophyll a and phycobilins) and are capable to oxygenic photosynthesis. Purple bacteria and green sulfur bacteria occupy habitats in the presence of hydrogen sulfide and oxidize and release sulfur gas rather than oxygen. Nitrogen fixing and denitrifying bacteria have an exceptionally important role in nitrogen cycling in terrestrial plant communities. Many vascular plants such as legumes and an aquatic fern have a symbiotic relationship with nitrogen fixing bacteria. Bacteria are also important carbon recyclers in the environment by assisting in the decomposition of dead organisms. Bacteria are considered Prokaryotes (before the nucleus) and represent a lineage that is ancient and possess a number of novel features. Bacteria generally lack internal compartmentalized organelles that are bound by membranes. Organelles such as Golgi bodies (dictyosomes), chloroplasts, mitochondria, and nuclei are regarded as unique to Eukaryotic (true nucleus) organisms. Recently, evolutionary biologists have determined that two major kingdoms of bacteria are present on the planet. Eubacteria represent the true bacteria and contain many of the groups that we are most familiar with such as cyanobacteria. E. coli, Streptococcus, and Rhizobium. Archaebacteria represent an ancient lineage whose members occupy rather inhospitable habitats such as deep sea thermal vents and miles deep in the Earth=s crust, but are also found in more normal environments. The objective of this lab is to examine the morphology, structure, and ecology of bacteria and cyanobacteria.