Memo for Vitamin D Analysis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Glee: Uma Transmedia Storytelling E a Construção De Identidades Plurais
UNIVERSIDADE FEDERAL DA BAHIA INSTITUTO DE HUMANIDADES, ARTES E CIÊNCIAS PROGRAMA MULTIDISCIPLINAR DE PÓS-GRADUAÇÃO EM CULTURA E SOCIEDADE ROBERTO CARLOS SANTANA LIMA GLEE: UMA TRANSMEDIA STORYTELLING E A CONSTRUÇÃO DE IDENTIDADES PLURAIS Salvador 2012 ROBERTO CARLOS SANTANA LIMA GLEE: UMA TRANSMEDIA STORYTELLING E A CONSTRUÇÃO DE IDENTIDADES PLURAIS Dissertação apresentada ao Programa Multidisciplinar de Pós-graduação, Universidade Federal da Bahia, como requisito parcial para obtenção do título de mestre em Cultura e Sociedade, área de concentração: Cultura e Identidade. Orientador: Prof. Dr. Djalma Thürler Salvador 2012 Sistema de Bibliotecas - UFBA Lima, Roberto Carlos Santana. Glee : uma Transmedia storytelling e a construção de identidades plurais / Roberto Carlos Santana Lima. - 2013. 107 f. Inclui anexos. Orientador: Prof. Dr. Djalma Thürler. Dissertação (mestrado) - Universidade Federal da Bahia, Faculdade de Comunicação, Salvador, 2012. 1. Glee (Programa de televisão). 2. Televisão - Seriados - Estados Unidos. 3. Pluralismo cultural. 4. Identidade social. 5. Identidade de gênero. I. Thürler, Djalma. II. Universidade Federal da Bahia. Faculdade de Comunicação. III. Título. CDD - 791.4572 CDU - 791.233 TERMO DE APROVAÇÃO ROBERTO CARLOS SANTANA LIMA GLEE: UMA TRANSMEDIA STORYTELLING E A CONSTRUÇÃO DE IDENTIDADES PLURAIS Dissertação aprovada como requisito parcial para obtenção do grau de Mestre em Cultura e Sociedade, Universidade Federal da Bahia, pela seguinte banca examinadora: Djalma Thürler – Orientador ------------------------------------------------------------- -

D-L Alvarez B
! D-L Alvarez b. 1966 Lives and works in Berlin, Germany Solo Exhibitions 2015 The Children’s Hour, [2nd Floor Projects], San Francisco, CA 2014 D-L Alvarez and Eileen Maxson, Artadia Gallery, New York, NY 2013 The Unforgiving Minute, Derek Eller Gallery, New York, NY 2012 D-L Alvarez: MATRIX 243, Berkley Art Museum, Berkley, CA 2011 Galeria Casado Santapau, Madrid, Spain 2009 Dusty Hayes, 2nd Floor Projects, San Francisco, CA 2008 Dead Leafs, Galeria Casado Santapau, Madrid, Spain 2007 Parents' Day, Derek Eller Gallery, New York, NY DIG, with Wayne Smith, Derek Eller Gallery (project room), New York, NY 2006 Casper, (with Matthew Lutz-Kinoy), A +B Arratiabeer, Berlin, Germany, 2005 rise, Derek Eller Gallery, New York, NY Ice, Glue, Berlin, Germany 2004 Beausoleil, Derek Eller Gallery, New York, NY 2002 The Road to Hell Less Traveled, Derek Eller Gallery, New York, NY 2000 Sculpture Garden, Derek Eller Gallery, New York, NY ! ! 1999 Chorus, John Berggruen Gallery, San Francisco, CA 1998 Knights Gathering Flowers, Derek Eller Gallery, New York, NY Dust, Th.e (Theoretical Events), Naples, Italy 1995 Dandylion, Jack Hanley Gallery, San Francisco, CA A Shepherd and His Flock, London Projects, London, UK 1994 Night of the Hunter, Kiki, San Francisco, CA 1990 Political Stance, (installation documenting performance), ATA, San Francisco, CA 1989 Elvis Clocked, Les Indes Galantes, Paris, France Group Exhibitions 2017 Drawings from the Collection: 1980 to Today, San Francisco Museum of Modern Art, San Francisco, CA 2016 Subject To Capital, Henry -

Kevin Mchale (Actor) Филм ÑпиÑък
Kevin McHale (actor) Филм ÑÐ ¿Ð¸ÑÑ ŠÐº (ФилмографиÑ) Rumours https://bg.listvote.com/lists/film/movies/rumours-2604427/actors Born This Way https://bg.listvote.com/lists/film/movies/born-this-way-2981342/actors https://bg.listvote.com/lists/film/movies/1065736/actors The Rocky Horror https://bg.listvote.com/lists/film/movies/the-rocky-horror-glee-show-2981329/actors Glee Show Showmance https://bg.listvote.com/lists/film/movies/showmance-2445773/actors Original Song https://bg.listvote.com/lists/film/movies/original-song-2600913/actors The Rhodes Not https://bg.listvote.com/lists/film/movies/the-rhodes-not-taken-2421156/actors Taken The Substitute https://bg.listvote.com/lists/film/movies/the-substitute-2982113/actors Acafellas https://bg.listvote.com/lists/film/movies/acafellas-517180/actors Special Education https://bg.listvote.com/lists/film/movies/special-education-2600265/actors Vitamin D https://bg.listvote.com/lists/film/movies/vitamin-d-2567406/actors Funeral https://bg.listvote.com/lists/film/movies/funeral-2604071/actors Duets https://bg.listvote.com/lists/film/movies/duets-2981365/actors Grilled Cheesus https://bg.listvote.com/lists/film/movies/grilled-cheesus-2981357/actors Furt https://bg.listvote.com/lists/film/movies/furt-2599821/actors The Break Up https://bg.listvote.com/lists/film/movies/the-break-up-1524605/actors The Role You Were https://bg.listvote.com/lists/film/movies/the-role-you-were-born-to-play-1704325/actors Born to Play Old Dog New Tricks https://bg.listvote.com/lists/film/movies/old-dog-new-tricks-16746534/actors -

Vitamin D in Milk T.M
South Dakota State University Open PRAIRIE: Open Public Research Access Institutional Repository and Information Exchange South Dakota State University Agricultural Bulletins Experiment Station 12-1-1935 Vitamin D in Milk T.M. Olson G.C. Wallis Follow this and additional works at: http://openprairie.sdstate.edu/agexperimentsta_bulletins Recommended Citation Olson, T.M. and Wallis, G.C., "Vitamin D in Milk" (1935). Bulletins. Paper 296. http://openprairie.sdstate.edu/agexperimentsta_bulletins/296 This Bulletin is brought to you for free and open access by the South Dakota State University Agricultural Experiment Station at Open PRAIRIE: Open Public Research Access Institutional Repository and Information Exchange. It has been accepted for inclusion in Bulletins by an authorized administrator of Open PRAIRIE: Open Public Research Access Institutional Repository and Information Exchange. For more information, please contact [email protected]. Experiment Bulletin 296 December 1935 Vitamin Din Milk T. M. Olson G. C. Wallis Rats Rats 1, 2 and 3 received Rachitogenic Ration supplemented with 12 cc of cows milk daily. Rat 4 received the Ratchitogenic Ration only. Dairy Department Agricultural Experiment Station South Dakota State College of Agriculture and Mechanic Arts Brookings, S. D. Table of Contents Page Introduction---------------------------------------------------- 3 Methods of estimating the amount of vitamin D ____________________ 3 Factors influencing the amount of vitamin D in the milk as produced by the cow----------------------------------------------------- 5 Vitamin D content under approximately normal conditions ____ 5 Effect of vitamin D during the growth of the animal __________ 6 Sunshine and seasonal effect------------------------------- 18 Ultraviolet irradiation of the cow--------------------------- 21 Feeding vitamin D concentrates --------------------------- 26 Breast feeding vs. -

Final Program
FINAL PROGRAM Contents Welcome . 2 Program Committee . 3 ACNM Leadership . 6 National Office Staff . 7 ACNM Fellows . 7 A .C .N .M . Foundation . 9 Meeting Information Schedule at a Glance . 12 Conference Center & Meeting Space . 14 Registration Services . 16 Exhibition Information . 17 General Meeting Services . 18 Business Meeting FAQs . 20 Business Meeting Procedures . 21 Business Meeting Agenda . 22 Awards . 23 Opening General Session . 25 Premier Sessions . 26 About CEUs . 28 Forums & Roundtables . 30 Posters . 32 Onsite Meetings . 34 Daily Programming Activities/Events, Workshops/Seminars, & Education Sessions Monday . 36 Tuesday . 37 Wednesday . 43 Thursday . 53 Friday . 61 Saturday . 71 Exhibition Exhibitors . 78 Exhibit Hall Map . 95 AMERICAN COLLEGE OF NURSE-MIDWIVES | 95 TH ANNUAL MEETING & EXHIBITION | DENVER, COLORADO | MAY 13–17, 2014 | WWW.MIDWIFE.ORG/AM | PAGE 1 Sample Running Head CONTINUED Welcome to the ACNM 59th Annual Meeting & Exhibition group, for our senior midwives . Don From the Program Chair your party regalia for the Awards On behalf of the 2014 National Program Dinner and Midwifery Celebration Party, and dance the night away Committee, I welcome you to the Mile High City! with the Hazel Miller Band . Stay ‘til the end to catch Saturday closing The program committees and national office staff have worked premier speaker Dawn Thompson of to make the ACNM 59th Annual Meeting & Exhibition in Denver, ImprovingBirth.org speaking about Colorado one not to be missed . Use your Final Program to learn forming a united front to improve the ins and outs of the sessions, customize your schedule based on maternity care . And as always, enjoy your areas of interest, and familiarize yourself with the layout of the the Heart of Midwifery gathering— Exhibit Hall . -

Graduate School of Biomedical Sciences
Graduate School of Biomedical Sciences 32nd Annual Student Research Week March 10-13, 2020 Texas Tech University Health Sciences Center (TTUHSC) Lubbock, Texas The Graduate School of Biomedical Sciences 2020 Student Research Week Committee Director: Bradley Schniers Vice Director of Marketing: Mariacristina Mazzitelli Vice Director of Poster Competition: Rachel Washburn Vice Director of Operations & Judging: Ryan Sweazey Website design and maintenance: Danny Boren, Graduate School of Biomedical Sciences Communications and social media: Suzanna Cisneros and Amy Skousen, Office of Communications Marketing; Leslie Fowler, Graduate School of Biomedical Sciences Speaker travel arrangements: Leslie Fowler, Graduate School of Biomedical Sciences Abstract book design: Deidra Satterwhite, Office of Student Life Student Research Week Banquet: Korac K, Graduate School of Biomedical Sciences Graduate Student Association; Velia Martinez, Graduate School of Biomedical Sciences The 2020 Student Research Week Committee would like to extend their warmest thanks to the following for their contributions and support in making Student Research Week a great success this year: The Graduate School of Biomedical Sciences staff: Leslie Fowler, Pam Johnson, Ashlee Rigsby and Velia Martinez The Office of Student Life: Deidra Satterwhite The Office of Communications and Marketing: Suzanna Cisneros, Amy Skousen and Kami Hunt The Office of the President: Bryce Looney The School of Medicine Office of the Dean: Charity Donaldson Educational Media Services: Neal Hinkle The departments of cell biology and biochemistry, pharmacology and neuroscience, immunology and molecular microbiology, cell physiology and molecular biophysics, medical education and graduate medical education; Graduate School of Biomedical Sciences at Lubbock, Abilene, and Amarillo, the School of Medicine, the School of Nursing, the School of Health Professions, the School of Pharmacy, the Office of Interprofessional Education, and Texas Tech University. -

Fantasizing Disability: Representation of Loss and Limitation in Popular Television and Film
Western University Scholarship@Western Electronic Thesis and Dissertation Repository 8-19-2014 12:00 AM Fantasizing Disability: Representation of loss and limitation in Popular Television and Film Jeffrey M. Preston The University of Western Ontario Supervisor Dr. Sharon Sliwinski The University of Western Ontario Graduate Program in Media Studies A thesis submitted in partial fulfillment of the equirr ements for the degree in Doctor of Philosophy © Jeffrey M. Preston 2014 Follow this and additional works at: https://ir.lib.uwo.ca/etd Part of the Feminist, Gender, and Sexuality Studies Commons, Film and Media Studies Commons, and the Television Commons Recommended Citation Preston, Jeffrey M., "Fantasizing Disability: Representation of loss and limitation in Popular Television and Film" (2014). Electronic Thesis and Dissertation Repository. 2386. https://ir.lib.uwo.ca/etd/2386 This Dissertation/Thesis is brought to you for free and open access by Scholarship@Western. It has been accepted for inclusion in Electronic Thesis and Dissertation Repository by an authorized administrator of Scholarship@Western. For more information, please contact [email protected]. FANTASIZING DISABILITY: REPRESENTATION OF LOSS AND LIMITATION IN POPULAR TELEVISION AND FILM (Monograph) by Jeffrey Preston Graduate Program in Media Studies A thesis submitted in partial fulfillment of the requirements for the degree of Doctorate in Media Studies The School of Graduate and Postdoctoral Studies The University of Western Ontario London, Ontario, Canada © Jeffrey Preston 2014 Abstract Most media texts currently being developed with disabled characters are crafted by individuals who are nondisabled and, as such, are based on what the nondisabled think it would be like to be disabled—a perception that is informed by the fantasy of disability. -
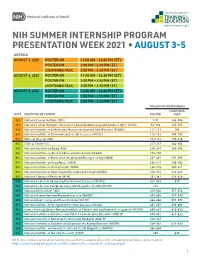
Summer Presentation Week
NIH SUMMER INTERNSHIP PROGRAM PRESENTATION WEEK 2021 • AUGUST 3-5 AGENDA AUGUST 3, 2021 POSTER AM 11:00 AM – 12:30 PM (ET) POSTER PM 1:00 PM – 2:30 PM (ET) LIGHTNING TALK 3:00 PM – 4:30 PM (ET) AUGUST 4, 2021 POSTER AM 11:00 AM – 12:30 PM (ET) POSTER PM 1:00 PM – 2:30 PM (ET) LIGHTNING TALK 3:00 PM – 4:30 PM (ET) AUGUST 5, 2021 POSTER AM 11:00 AM – 12:30 PM (ET) POSTER PM 1:00 PM – 2:30 PM (ET) LIGHTNING TALK 3:00 PM – 4:30 PM (ET) PRESENTATION NUMBERS LIGHTNING DATE INSTITUTE OR CENTER POSTER TALK 8/3 National Cancer Institute (NCI) 1-91 144-184 8/3 National Cancer Institute – Division of Cancer Epidemiology and Genetics (NCI – DCEG) 92-110 185-187 8/3 National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) 111-113 188 8/3 National Institute of Environmental Health Sciences (NIEHS) 114-141 189-192 8/3 Office of Director (OD) 142-143 193-216 8/4 Clinical Center (CC) 217-237 362-364 8/4 National Institute on Aging (NIA) 238-247 365-396 8/4 National Institute on Alcohol Abuse and Alcoholism (NIAAA) 248-256 8/4 National Institute of Biomedical Imaging and Bioengineering (NIBIB) 257-261 397-399 8/4 National Institute on Drug Abuse (NIDA) 262-279 400-402 8/4 National Institute of Mental Health (NIMH) 280-315 403-411 8/4 National Institute of Neurological Disorders and Stroke (NINDS) 316-351 412-423 8/4 National Library of Medicine (NLM) 352-361 424-426 8/5 National Center for Advancing Translational Sciences (NCATS) 427-433 570 8/5 National Center for Complementary and Integrative Health (NCCIH) 434 8/5 National Eye Institute -

Кð¾ñ€Ð¸ Ðœð¾ð½ñ‚ийñ‚ Филм ÑпиÑÑšðº (ФÐ
Кори Монтийт Филм ÑÐ ¿Ð¸ÑÑ ŠÐº (ФилмографиÑ) Rumours https://bg.listvote.com/lists/film/movies/rumours-2604427/actors Born This Way https://bg.listvote.com/lists/film/movies/born-this-way-2981342/actors https://bg.listvote.com/lists/film/movies/1065736/actors The Rocky Horror https://bg.listvote.com/lists/film/movies/the-rocky-horror-glee-show-2981329/actors Glee Show Showmance https://bg.listvote.com/lists/film/movies/showmance-2445773/actors Original Song https://bg.listvote.com/lists/film/movies/original-song-2600913/actors The Rhodes Not https://bg.listvote.com/lists/film/movies/the-rhodes-not-taken-2421156/actors Taken The Substitute https://bg.listvote.com/lists/film/movies/the-substitute-2982113/actors Acafellas https://bg.listvote.com/lists/film/movies/acafellas-517180/actors Special Education https://bg.listvote.com/lists/film/movies/special-education-2600265/actors Vitamin D https://bg.listvote.com/lists/film/movies/vitamin-d-2567406/actors Funeral https://bg.listvote.com/lists/film/movies/funeral-2604071/actors Duets https://bg.listvote.com/lists/film/movies/duets-2981365/actors Grilled Cheesus https://bg.listvote.com/lists/film/movies/grilled-cheesus-2981357/actors Furt https://bg.listvote.com/lists/film/movies/furt-2599821/actors The Break Up https://bg.listvote.com/lists/film/movies/the-break-up-1524605/actors The Role You Were https://bg.listvote.com/lists/film/movies/the-role-you-were-born-to-play-1704325/actors Born to Play Mademoiselle C. https://bg.listvote.com/lists/film/movies/mademoiselle-c.-16574792/actors -

Vitamin D Status of Athletes with High UV-Exposure During Exercise Training1
Author's copy! Any use beyond the limits of copyright law without the consent of the publisher is prohibited and punishable. This applies in particular to duplications, translations, microfilming as well as storage and processing in electronic systems. Science & Research | Original Contribution Peer-reviewed | Manuscript received: February 26, 2013 | Revision accepted: July 18, 2013 Vitamin D status of athletes with high UV-exposure during exercise training1 Anja Carlsohn, Friederike Scharhag-Rosenberger, Juliane Heydenreich, Frank Mayer, Potsdam cle protein synthesis and neuromus- Summary cular control [7–9]. It has therefore been suggested that vitamin D sta- The summer vitamin D status was measured of 35 professional and recreatio- tus and physical performance may nal athletes. Professional and recreational athletes consumed 3.5 ± 5.9 g/d and be linked and this has been supported 3.9 ± 5.1 g/d vitamin D, respectively (p = 0.82). However, the serum values by some studies [1, 10, 11]. How- (90 ± 14 nmol/L and 74 ± 2 nmol/L; p = 0.03) were within the normal range. ever, recent investigations have It is possible that UV exposure during training makes a major contribution to- shown that young athletes – partic- wards meeting requirements. ularly in indoor sports – may exhibit Keywords: Vitamin D status, nutrition, sports, excercise inadequate or low serum concentra- tions of 25-(OH)-cholecalciferol [13, 14]. Introduction corresponding to 80–90 % of vitamin D supply [1, 2]. Objective The fat soluble vitamin D is essential A deficient vitamin D status is re- for intra- and extracellular potas- The objective of this study was to flected by the serum concentration sium and phosphate homeostasis, as record vitamin D intake and the of 25-(OH)-cholecalciferol, as 25- well as for bone mineralisation. -

Update on Vitamins and Minerals & the RD Scope of Practice Page 4
2 résumé AN EXCEPTIONAL OPPORTUNITY TO SERV E Update on Vitamins and Minerals & 3 page 4 UNDERSTANDING THE the RD Scope of Practice RIGHT OF CLIENTS TO MAKE AN INFORMED DECISION 8 New Obligations for Reporting SHARING PERSONAL Page 10 HEALTH INFORMATION Privacy Breaches WITHIN THE CIRCLE OF CARE 11 DISPELLING MYTHS OF A Voluntary Undertaking is a Legally THE COLLEGE’S PROFESSIONAL Binding Agreement page 14 PRACTICE ADVISORY SERVICE 16 Register Online Now! HOW TO CHANGE YOUR NAME IN THE CDO 2016 WORKSHOP COLLEGE PROFILE Schedule Back Cover www.collegeofdietitians.org SUMMER 2016 PRESIDENT’S MESSAGE An Exceptional Opportunity to Serve I am honoured to be addressing you as the new President of the College. I am a Clinical Dietitian working in an acute care facility in Northwestern Ontario and have been an elected Council member for the past four years including one term on the Executive Committee. I am currently also Chair of the Inquiries, Complaints and Reports Committee and have served as a member of the Quality Assurance, Legislative Issues, and Patient Relations Committees. Erin Woodbeck, RD This is a particularly exciting time to be involved in a leadership role on College President Council. We have nearly completed our first full year under the guidance of our new Registrar & Executive Director and have launched a new four year strategic plan. The The College of Dietitians of Council members are a very diverse and engaged group of public and elected Council Ontario is dedicated to members who bring unique perspectives to all issues discussed. public protection. The position of President is clearly an exceptional opportunity for professional We regulate and support development with a particular emphasis on enhancing leadership skills. -
2021Commencementprogram1.Pdf
One Hundred and Sixty-Third Annual Commencement JUNE 14, 2021 One Hundred and Sixty-Third Annual Commencement 11 A.M. CDT, MONDAY, JUNE 14, 2021 UNIVERSITY SEAL AND MOTTO Soon after Northwestern University was founded, its Board of Trustees adopted an official corporate seal. This seal, approved on June 26, 1856, consisted of an open book surrounded by rays of light and circled by the words North western University, Evanston, Illinois. Thirty years later Daniel Bonbright, professor of Latin and a member of Northwestern’s original faculty, redesigned the seal, Whatsoever things are true, retaining the book and light rays and adding two quotations. whatsoever things are honest, On the pages of the open book he placed a Greek quotation from the Gospel of John, chapter 1, verse 14, translating to The Word . whatsoever things are just, full of grace and truth. Circling the book are the first three whatsoever things are pure, words, in Latin, of the University motto: Quaecumque sunt vera whatsoever things are lovely, (What soever things are true). The outer border of the seal carries the name of the University and the date of its founding. This seal, whatsoever things are of good report; which remains Northwestern’s official signature, was approved by if there be any virtue, the Board of Trustees on December 5, 1890. and if there be any praise, The full text of the University motto, adopted on June 17, 1890, is think on these things. from the Epistle of Paul the Apostle to the Philippians, chapter 4, verse 8 (King James Version).