Gas Generator Bottle Introduction SCIENTIFIC This Gas Generator Setup Provides an Easy Way to Generate and Collect Gas
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Oil Analysis Handbook Third Edition Copyright © 2017 Spectro Scientific
Predictive Equipment Maintenance Oil Analysis Handbook Third Edition Copyright © 2017 Spectro Scientific. All rights reserved. FluidScan® and LaserNet Fines® are registered trademarks of Spectro Scientific Inc. While every effort is made to assure the information in this document ois accurate, Spectro Scientific does not accept liability for any errors or mistakes that may arise. Specifications are subject to change without notice. | 3 Preface Welcome to the third edition of the In-service Oil Analysis Handbook. It has been a few years since the publication of the first edition of Spectro Scientific’s In-Service Oil Analysis Handbook. Our original goal was to compile a comprehensive reference book of common in-service oil analysis techniques to help readers understand and choose the right technique and instrumentation for their needs. We had to limit the scope in the first two editions because of the amount of effort needed to cover all the topics. In-service oil analysis for condition based maintenance covers a wide array of topics. I am pleased to say that in this third edition, we are much closer to our goal. We reorganized the structure of the content for better clarity and we added articles to cover more topics and instruments associated with oil analysis. Also, we rewrote several articles including the latest developments on the market. As we learned more from our customers about their successes using on site oil analysis, we developed case studies that you can find in this edition. This work is not possible without the time and effort from the contributing authors: Patrick Henning, Daniel Walsh, Robert Yurko, Ken Caldwell, Thomas Barraclough, Maria Bartus, Randi Price, John Morgan, Aifeng Shi and Yuegang Zhao from Spectro Scientific and Ray Garvey from Emerson Process Management. -

Food Packaging (FS 522 / FS 495) Aseptic Processing and Packaging
Food Packaging (FS 522 / FS 495) Aseptic Processing and Packaging Components of an aseptic processing system Aseptic processing is a thermal process in which the product and container are sterilized separately and brought together in a sterile environment. It involves pumping, deaeration, and sterilization of a food product, followed by holding it for a specified period of time (in a holding tube -- required to have a 1/4" rise per foot length of tube), cooling it, and finally packaging it in a sterile container. The use of high temperature for a short period of time (in comparison with conventional canning) in aseptic processing yields a high quality product. Care should be taken to ensure that all process calculations are performed after the deaeration stage and not based on the initial raw product. Deaeration is accomplished in a vessel maintained at a certain degree of vacuum by means of a vacuum pump. The product is fed into the vessel at 55 - 70 /C through a nozzle at the center of the vessel. Vacuum is controlled to obtain a product flash of about 5 /C. An internal spiral condenser condenses vapors and other condensable gases. The deaerated product is discharged through the bottom and pumped to the heating section. Another important part of an aseptic processing system is the back pressure valve which provides sufficient pressure to prevent boiling of the product at processing temperatures which can be as high as 125-130 /C. An aseptic surge tank provides the means for product to be continuously processed even if the packaging system is not operational due to any malfunction. -

15.0 Hazardous Waste Disposal
LABORATORY SAFETY MANUAL Revised June 2018 Emergency Contact Information In case of emergency: 911 Security Services: (daytime) 705-474-7600 ext. 5555 (cell phone) 705-498-7244 Laboratory Safety Coordinator: 474-3450 ext. 4180 Emergency Procedures** Chemical Spill On Body • Rinse affected area immediately for 15 minutes using emergency shower, if required. • Care must be taken to avoid contamination with face and eye(s). In Eye(s) • Immediately flush eye(s) using emergency eyewash station for a minimum of 15 minutes. In Laboratory • Assess the scene. If the situation is beyond your capabilities, contact your supervisor. • If safe to do so, turn off any ignition sources if flammable material is present. • Use spill kits to assist in spill containment. • Dispose of waste material with other hazardous waste. Fire • If you hear the Fire Alarm ring continuously evacuate the building immediately. • If you hear the Fire Alarm ring intermittently be alert however evacuation is not necessary. • If Fire Alarm changes from intermittent to continuous evacuate immediately • If you detect a fire, do not attempt to extinguish it yourself unless you are capable and it is safe to do so. Report the injury/incident to your supervisor as soon as possible. ** This is to be used as a quick reference only. For more detailed information, please refer to section 8.0 Emergency Procedures. Laboratory Safety Manual – Nipissing University 2 Table of Contents Acknowledgements ................................................................................................... -

Trace Elements Specimen Collection Guide
500 Chipeta Way Salt Lake City, Utah 84108-1221 Phone: 801-583-2787 | toll free: 800-242-2787 A nonprofit enterprise of the University of Utah and its Department of Pathology Fax: 801-522-2706 | aruplab.com TRACE ELEMENTS SPECIMEN COLLECTION GUIDE Analysis for trace elements at ARUP is performed in a clean laboratory environment that includes a system of positive- pressure and HEPA-filtered air. Since detection limits for many trace elements are calculated in parts per billion, this helps minimize environmental contamination of specimens. Additionally, contamination control during specimen collection provides more accurate and clinically useful results. Note: Refer to the ARUP Laboratory Test Directory for specific instructions when collecting specimens for cadmium exposure testing (http://ltd.aruplab.com/Tests/Pub/0025013). Urine Collection Tissue Although it is nearly impossible to obtain a completely Follow information on individual pages of the laboratory uncontaminated urine specimen, steps can be taken to test directory. minimize environmental contamination. Avoid collecting the specimen in an area where Whole Blood, Serum, and Red Blood Cell Collection Materials comprising the tube and the tube top are a environmental contamination is likely to occur. In an consideration. Rubber stoppers in collection tubes other industrial or construction setting, it is important that than the royal blue tube are known to contain elements clothing worn in the workplace be removed prior to that can contaminate a specimen. Contamination can be specimen collection to prevent dust on the clothing introduced as the needle punctures the stopper in a tube from contaminating the specimen. other than royal blue. Most of this also holds true for Wash and dry hands thoroughly prior to collection. -

Rigid Intermediate Bulk Containers: United States
Freedonia Focus Reports US Collection Rigid Intermediate Bulk Containers: United States February 2019 CLICK TO ORDER FULL REPORT BROCHURE www.freedoniafocusreports.com CLICK TO ORDER FULL REPORT Table of Contents 1. Highlights 3 2. Market Environment 4 Historical Trends 4 Key Economic Indicators 5 Competition from Reconditioned & Refurbished Packaging 6 Regulatory Factors 8 3. Segmentation & Forecasts 10 Products 10 Plastic Body 11 Metal Body 12 Other Rigid Intermediate Bulk Containers 13 Markets 14 Chemicals & Pharmaceuticals 15 Food & Beverages 15 Petroleum & Lubricants 16 Hazardous Waste 16 Agricultural & Horticultural Products 16 Plastic & Rubber 17 Other Markets 17 4. Industry Structure 19 Industry Characteristics 19 Market Leaders 20 Mauser Packaging Solutions 20 SCHÜTZ 21 Greif 21 5. About This Report 22 Scope 22 Sources 22 Industry Codes 23 Freedonia Methodology 23 Resources 25 Rigid Intermediate Bulk Containers: United States 1 ©2019 The Freedonia Group. All rights reserved. List of Tables & Figures Figure 1 | Key Trends in the US Rigid Intermediate Bulk Container Market, 2018 – 2023 3 Figure 2 | US Rigid Intermediate Bulk Container Demand Trends, 2008 – 2018 4 Table 1 | Key Indicators for US Rigid Intermediate Bulk Container Demand, 2008 – 2023 (US$ bil) 5 Figure 3 | US Rigid Intermediate Bulk Container Demand by Product, 2008 – 2023 (US$ mil) 10 Table 2 | US Rigid Intermediate Bulk Container Demand by Product, 2008 – 2023 (US$ mil) 10 Figure 4 | US Rigid Intermediate Bulk Container Demand by Product, 2008 – 2023 (%) 12 Figure 5 | US Rigid Intermediate Bulk Container Demand by Market, 2008 – 2023 (US$ mil) 14 Table 3 | US Rigid Intermediate Bulk Container Demand by Market, 2008 – 2023 (US$ mil) 14 Figure 6 | US Rigid Intermediate Bulk Container Demand by Market, 2008 – 2023 (%) 18 Table 4 | Leading Suppliers in the US Rigid Intermediate Bulk Container Market by Product 20 Table 5 | NAICS & SIC Codes Related to Rigid Intermediate Bulk Containers 23 Rigid Intermediate Bulk Containers: United States 2 ©2019 The Freedonia Group. -

Nalgene and Nunc Centrifuge Ware Catalog
Nalgene and Nunc Centrifuge Ware Select the right vessel and spin with confidence Spin with confidence at virtually any scale The process of selecting a centrifuge and rotor can feel like the easy part when faced with choosing the tube or bottle that is the right fit for both the rotor and application. There are several factors to consider when selecting the correct vessel for each application: • Chemical compatibility • Volume • Temperature • Relative centrifugal force (RCF) required • Protocols to be used for loading and sample recovery • Cleaning and autoclaving steps Understanding your requirements before selecting a tube or bottle ensures you make the right choice. Whether your application includes the need for separations, large volume pelleting, protein purification or DNA isolation, the comprehensive selection of Thermo Scientific™ Nalgene™ and Thermo Scientific™ Nunc™ centrifuge ware offers a solution for virtually scales and is available in sizes from 10 mL to 2 L. Such a broad offering means a tube or bottle for many spins – from clinical and bioproduction, to processing bacteria, yeast, tissue, and viruses. Contents Centrifuge tubes 4 Centrifuge bottles 20 Closures and adaptors 31 Resources 36 Simplify performance at every turn with a reliable and safe approach to centrifugation Nalgene Conical-Bottom Centrifuge Tubes polypropylene copolymer Thermo Scientific™ Nalgene™ PPCO conical-bottom centrifuge tubes with molded-in graduations have excellent chemical resistance. Designed for low-speed centrifugation in refrigerated and non-refrigerated centrifuges details • Translucent PPCO is compatible with a wide range of lab reagents • Conical bottoms concentrate pellet in a small area for easy isolation and retrieval • Molded-in graduations last the life of the tube • Last longer than polycarbonate tubes under conditions of repeated Note: Centrifuge tubes must be filled at least 80% for proper performance. -

K–12 Science Safety Manual
K–12 Science Safety Manual Developed by the Innovative Teaching and Learning Unit Instructional Design and Professional Learning Division K–12 Science Department TABLE OF CONTENTS Importance of Safety 2 General Information 7 Prohibited Practices 9 Emergency Information 10 Safety on Field Trips 15 General Laboratory Safety 20 Elementary Science Safety 22 Biological Science Safety 24 Earth and Space Science Safety 28 Chemistry Science Safety 31 Physics Science Safety 35 Appendix A: Technical Safety Assistance Numbers 49 Appendix B: Safety Survey 50 Appendix C: Lab Inspection Checklist 51 Appendix D: Chemical Storage 55 Appendix E: Waste Disposal 63 Appendix F: Prohibited Chemicals 68 Appendix G: Restricted Chemicals 75 Appendix H: Animals in the Classroom 79 Appendix I: Safety Contracts and Exams 85 Appendix J: Safety Resource Websites 86 Restricted Chemical Request Form 87 Secondary Science Teacher Safety Manual Acknowledgement Form 88 IDPL-SCI-M002, Rev. B September 2009, Rev. C April 2015 0 SPECIAL ACKNOWLEDGMENTS The Clark County School District K–12 Science Safety Manual is a coordinated effort by a team of teachers, administrators, with input from the Southern Nevada Health District. Andy Cheney Environmental Health Supervisor, Southern Nevada Health District Ellen Dunne K–5 Science Project Facilitator, CCSD Instructional Design and Professional Learning Division Eileen Gilligan Elementary Science Coordinator, CCSD Instructional Design and Professional Learning Division Kim Krumland Risk and Environmental Services Department Director, -
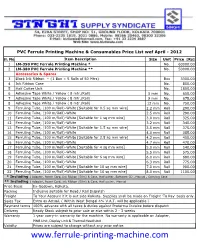
Ferrule Printing Machine & Consumables Price List Wef April - 2012
PVC Ferrule Printing Machine & Consumables Price List wef April - 2012 Sl. No. Item Description Size Unit Price [Rs] 1 LM-390 PVC Ferrule Printing Machine * No. 60000.00 2 LM-380 PVC Ferrule Printing Machine ** No. 50000.00 Accessories & Spares 3 Black Ink Ribbon – {1 Box = 5 Rolls of 50 Mtrs} Box 3300.00 4 Ink Ribbon Case No. 850.00 5 Half Cutter Unit No. 1800.00 6 Adhesive Tape White / Yellow ( 8 mtr./Roll) 5 mm No. 650.00 7 Adhesive Tape White / Yellow ( 8 mtr./Roll) 9 mm No. 675.00 8 Adhesive Tape White / Yellow ( 8 mtr./Roll) 12 mm No. 750.00 9 Ferruling Tube, (100 m/Roll)-White [Suitable for 0.5 sq mm wire] 2.2 mm Roll 290.00 10 Ferruling Tube, (100 m/Roll)-White 2.5 mm Roll 290.00 11 Ferruling Tube, (100 m/Roll)-White [Suitable for 1 sq mm wire] 3.0 mm Roll 325.00 12 Ferruling Tube, (100 m/Roll)-White 3.2 mm Roll 340.00 13 Ferruling Tube, (100 m/Roll)-White [Suitable for 1.5 sq mm wire] 3.5 mm Roll 375.00 14 Ferruling Tube, (100 m/Roll)-White 4.0 mm Roll 385.00 15 Ferruling Tube, (100 m/Roll)-White [Suitable for 2.5 sq mm wire] 4.2 mm Roll 450.00 16 Ferruling Tube, (100 m/Roll)-White 4.7 mm Roll 470.00 17 Ferruling Tube, (100 m/Roll)-White [Suitable for 4 sq mm wire] 5.0 mm Roll 540.00 18 Ferruling Tube, (100 m/Roll)-White 5.5 mm Roll 575.00 19 Ferruling Tube, (100 m/Roll)-White [Suitable for 6 sq mm wire] 6.0 mm Roll 680.00 20 Ferruling Tube, (100 m/Roll)-White 6.3 mm Roll 775.00 21 Ferruling Tube, (100 m/Roll)-White [Suitable for 10 sq mm wire] 7.0 mm Roll 900.00 22 Ferruling Tube, (100 m/Roll)-White [Suitable for 16 sq mm wire] 8.0 mm Roll 1100.00 * Including : Adapter, Power Cord, Ink Ribbon (50m) & Case, Half cutter, Software CD , Manual , Carrying Case ** Including : Adapter, Power Cord, Ink Ribbon (50m) & Case, Half cutter, Manual Price Basis Ex-Godown, Kolkata. -

Chemistry 1009 Lab Manual University of Louisiana at Monroe
Chemistry 1009 Lab Manual University of Louisiana at Monroe Department of Chemistry 2010 Version 2.0 Contents Lab Session 1: Laboratory Safety Rules and Check In .................................................. 1 Fire, Injury, Spills and Cleanliness .............................................................................. 2 Desk Assignment Sheet (Chemistry 1009) .................................................................. 4 Commonly Used Equipment (not in the desk drawer)................................................. 5 Lab Session 2, Experiment 1: Introductory Exercises ................................................... 10 Report Form 1 .............................................................................................................. 17 Lab Session 3, Experiment 2: Oxygen ........................................................................... 18 Report Form 2 .............................................................................................................. 21 Lab Session 4, Experiment 3: Preparation of Sodium Chloride .................................... 22 Report Form 3 .............................................................................................................. 25 Lab Session 5, Experiment 4: Law of Definite Proportions .......................................... 26 Report Form 4 ........................................................................................................... 30 Lab Session 6, Experiment 5: Hydrogen and the Activity Series of Metals ................. -

Fisherbrand Reusable Glassware Brochure
Glassware Disposable, Reusable and Specialty Table of Contents Glassware Beakers ............................................................................................................................................................................................................... 1 Bottles ..............................................................................................................................................................................................................1-2 Media ........................................................................................................................................................................................................... 1 Storage .....................................................................................................................................................................................................1-2 Burets ...............................................................................................................................................................................................................2-3 Cells ..................................................................................................................................................................................................................4-5 Spectrophotometer ................................................................................................................................................................................. -

The Calibration of Small Volumetric Laboratory Glassware
NBSIR 74-461 The Calibration of Small Volumetric Laboratory Glassware Josephine Lembeck Institute for Basic Standards National Bureau of Standards Washington, D. C. 20234 December 1974 Final Prepared for Optical Physics Division Institute for Basic Standards Washington, D. C. 20234 NBSIR 74-461 THE CALIBRATION OF SMALL VOLUMETRIC LABORATORY GLASSWARE Josephine Lembeck Institute for Basic Standards National Bureau of Standards Washington, D. C. 20234 December 1974 Final Prepared for Optical Physics Division Institute for Basic Standards Washington, D. C. 20234 U. S. DEPARTMENT OF COMMERCE, Frederick B. Dent, Secretary NATIONAL BUREAU OF STANDARDS, Richard W. Roberts. Director The Calibration of Small Volumetric Laboratory Glassware by Josephine Lembeck 1. INTRODUCTION , This article describes an acceptable procedure for calibrating burets, pipets and volumetric flasks of the types shown in figure 1. The physical characteristics of these items are indicated in Interim Federal Specifications NNN-B-00789a (GSA-FSS) , NNN-P- 00395a (GSA-FSSi NNN-F-00289a (GSA-FSS), and NBS Handbook 105-2. The general procedure is based upon a determination of the volume of water either contained in or delivered by the vessel. This volume of water is based upon knowledge of its measured mass and its tabulated density. Inasmuch as these calibration volumes, especially the delivery volume, may be altered by the manner in which the vessels are used, it is imperative that the user of the vessel employ the same techniques as those employed during calibration. The techniques of importance here are: 1. reading and adjusting meniscus 2. filling the vessel 3. draining the vessel. v These three topics will be covered in detail. -

Urinary Drainage Bottle Urine Collection System
URINARY DRAINAGE BOTTLE URINE COLLECTION SYSTEM FOR USE WITH FOLEY INDWELLING CATHETERS, MALE EXTERNAL CATHETERS AND UROSTOMY APPLICATIONS USE AND CARE INSTRUCTIONS FOR PRODUCT #4100 GH-F HI LO us W o 13 L i m t a t F l e r R u e o d s V e r a l u 410002 v t e a ™ e F LATEX-FREE DEHP-FREE KIT CONTENTS: 1 Durable Urinary Drainage Bottle (#410001) 1 Bottle Cap with Housing (#4101) 1 60" (1.5m) Drainage Tube with Adaptor (#6061) 1 Adjustable Tube Clamp (#6999) 1 Urinary Drainage Bottle Adaptor (#6003) 1 “HIGH-FLOW” Red Anti-Reflux Valve (#6001) 1 Large Gasket-Ring (#6000) 1 Pair of Hooks for Hanging (#4102) 1 Illustrated Use and Care Instructions CAUTION: If red anti-reflux valve is missing or removed, leaks around the top valve housing may occur. The use of Gasket-Ring (#6000) will be necessary to seal the housing threads and prevent leaking. #6003 Urinary Drainage Bottle Adaptor #6061 Clear-Vinyl Drainage Tube with Graduated Adaptor and Cap # 6000 Black Gasket-Ring # replace for anti-reflux valve 13 6001 High-Flow #6999 Adjustable when not in use. Anti-Reflux Valve Thumb Clamp IMPORTANT: To avoid drainage problems, before using this product, read all instructions #6003 Urinary Drainage and information carefully. 13 Bottle Adaptor Understand how it works and how to maintain it properly. #6001 High-Flow #4101 Anti-Reflux Valve 13 Urinary Drainage FIGURE 2 Bottle Cap #4101 TOP VALVE HOUSING DETAIL Urinary Drainage Bottle Cap #6003 Urinary Drainage Bottle Adaptor #603005 Top Valve Housing (not sold separately) #4102 Urinary Drainage Bottle Hooks # 410001 Drainage Bottle (not sold separately) 410002 #410001 Urinary Drainage Bottle (not sold separately) FIGURE 3 BOTTLE CAP CLOSE-UP To remove adaptor (#6003) from bottle cap, remove tubing (#6061), hold housing (#603005) in place and turn adaptor counter-clockwise.