UNIVERSITY of CALIFORNIA Los Angeles Configuration of a Molten
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Preliminary Report of the Battle of the Crater, 30 July 1864
Holding the Line A Preliminary Report of The Battle of the Crater 30 July 1864 Adrian Mandzy, Ph. D. Michelle Sivilich, Ph. D. Benjamin Lewis Fitzpatrick, Ph. D. Dan Sivilich Floyd Patrick Davis Kelsey P. Becraft Dakota Leigh Goedel Jeffrey A. McFadden Jessey C. Reed Jaron A. Rucker A PRELIMINARY REPORT ON THE SURVEY OF THE BATTLE OF THE CRATER, 30 JULY 1864 By Adrian Mandzy, Ph.D., Michelle Sivilich, Ph. D., Floyd Patrick Davis, Kelsey P. Becraft, Dakota Leigh Goedel, Jeffrey A. McFadden, Jessey C. Reed, and Jaron A. Rucker With a Contributions by Daniel Sivilich and Dr. Benjamin Lewis Fitzpatrick Report prepared for the Northeast Region Archeology Program National Park Service 115 John Street, 4th Floor Lowell, Massachusetts 01852-1195 _______________________________ Adrian Mandzy Principal Investigator ARPA Permit 2014.PETE.01 2 Abstract In March 2015, faculty and students from Morehead State University’s History program, along with members of the Battlefield Restoration and Archeological Volunteer Organization (BRAVO) conducted a survey of The Crater Battlefield. Fought on 30 July 1864, during the Siege of Petersburg, the Battle of the Crater, according to the National Park Service, is one of the most important events of the Civil War. The participation of African-American troops in the battle and the subsequent execution of black prisoners highlights the racial animosities that were the underpinning causes of this conflict. The goal of this project is to document the level of integrity of any archaeological resources connected with this field of conflict and to examine how far the Union troops advance beyond the mouth of the Crater. -

Confronting Antisemitism in Modern Media, the Legal and Political Worlds an End to Antisemitism!
Confronting Antisemitism in Modern Media, the Legal and Political Worlds An End to Antisemitism! Edited by Armin Lange, Kerstin Mayerhofer, Dina Porat, and Lawrence H. Schiffman Volume 5 Confronting Antisemitism in Modern Media, the Legal and Political Worlds Edited by Armin Lange, Kerstin Mayerhofer, Dina Porat, and Lawrence H. Schiffman ISBN 978-3-11-058243-7 e-ISBN (PDF) 978-3-11-067196-4 e-ISBN (EPUB) 978-3-11-067203-9 DOI https://10.1515/9783110671964 This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. For details go to https://creativecommons.org/licenses/by-nc-nd/4.0/ Library of Congress Control Number: 2021931477 Bibliographic information published by the Deutsche Nationalbibliothek The Deutsche Nationalbibliothek lists this publication in the Deutsche Nationalbibliografie; detailed bibliographic data are available on the Internet at http://dnb.dnb.de. © 2021 Armin Lange, Kerstin Mayerhofer, Dina Porat, Lawrence H. Schiffman, published by Walter de Gruyter GmbH, Berlin/Boston The book is published with open access at www.degruyter.com Cover image: Illustration by Tayler Culligan (https://dribbble.com/taylerculligan). With friendly permission of Chicago Booth Review. Printing and binding: CPI books GmbH, Leck www.degruyter.com TableofContents Preface and Acknowledgements IX LisaJacobs, Armin Lange, and Kerstin Mayerhofer Confronting Antisemitism in Modern Media, the Legal and Political Worlds: Introduction 1 Confronting Antisemitism through Critical Reflection/Approaches -

Muslim Scientists and Thinkers
MUSLIM SCIENTISTS AND THINKERS Syed Aslam Second edition 2010 Copyright 2010 by Syed Aslam Publisher The Muslim Observer 29004 W. Eight Mile Road Farmington, MI 48336 Cover Statue of Ibn Rushd Cordoba, Spain ISBN 978-1-61584-980-2 Printed in India Lok-Hit Offset Shah-e-Alam Ahmedabad Gujarat ii Dedicated to Ibn Rushd and other Scientists and Thinkers of the Islamic Golden Age iii CONTENTS Acknowledgments ................................................VI Foreword .............................................................VII Introduction ..........................................................1 1 Concept of Knowledge in Islam ............................8 2 Abu Musa Jabir Ibn Hayyan..................................25 3 Al-Jahiz abu Uthman Ibn Bahar ...........................31 4 Muhammad Ibn Musa al-Khwarizmi....................35 5 Abu Yaqoub Ibn Ishaq al-Kindi ............................40 6 Muhammad bin Zakaria Razi ...............................45 7 Jabir ibn Sinan al-Batani.......................................51 8 Abu Nasar Mohammad ibn al-Farabi....................55 9 Abu Wafa ibn Ismail al-Buzjani ...........................61 10 Abu Ali al-Hasan ibn al-Haytham .......................66 11 Abu Rayhan ibn al-Biruni ....................................71 12 Ali al-Hussain ibn Sina ........................................77 13 Abu Qasim ibn al-Zahrawi ..................................83 iv 14 Omar Khayyam ...................................................88 15 Abu Hamid al-Ghazali .........................................93 -
Diamond Craters Oregon's Geologic
Text by Ellen M. Benedict, 1985 Features at stops correspond to points on a clock ago, a huge mass of hot gases, volcanic ashes, bits face. Imagine that you are standing in the middle of a of pumice and other pyroclastics (fire-broken rock) Travel And Hiking Hints clock face. Twelve o’clock is the road in front of you violently erupted. The blast – greater than the May and 6 o’clock the road behind. If you always align the 18, 1980, eruption of Mt. St. Helens – deposited a Diamond Craters is located in the high desert country clock face with the road, you should be able to locate layer of pyroclastics 30 to 130 feet thick over an area about 55 miles southeast of Burns, Oregon. It’s an the features. almost 7,000 square miles! isolated place and some precautions should be taken . when traveling in the area. Start Tour. Mileage begins halfway Pyroclastics are between milepost 40 and 41 on State normal behavior Diamond Craters has no tourist facilities. The nearest Highway 205 at the junction to Diamond. for magmas place where gasoline is sold is at Frenchglen. Turn left. (subsurface That’s the opinion held by scores of molten rocks) Keep your scientists and educators who have visited Diamond, Oregon, a small ranching community, was of rhyolitic (a vehicle on named in 1874 for Mace McCoy’s Diamond brand. volcanic material and studied the area. It has the “best and hard-packed The nearby craters soon became known as Diamond related to granite) most diverse basaltic volcanic features in the road surfaces Craters. -

Appendix I Lunar and Martian Nomenclature
APPENDIX I LUNAR AND MARTIAN NOMENCLATURE LUNAR AND MARTIAN NOMENCLATURE A large number of names of craters and other features on the Moon and Mars, were accepted by the IAU General Assemblies X (Moscow, 1958), XI (Berkeley, 1961), XII (Hamburg, 1964), XIV (Brighton, 1970), and XV (Sydney, 1973). The names were suggested by the appropriate IAU Commissions (16 and 17). In particular the Lunar names accepted at the XIVth and XVth General Assemblies were recommended by the 'Working Group on Lunar Nomenclature' under the Chairmanship of Dr D. H. Menzel. The Martian names were suggested by the 'Working Group on Martian Nomenclature' under the Chairmanship of Dr G. de Vaucouleurs. At the XVth General Assembly a new 'Working Group on Planetary System Nomenclature' was formed (Chairman: Dr P. M. Millman) comprising various Task Groups, one for each particular subject. For further references see: [AU Trans. X, 259-263, 1960; XIB, 236-238, 1962; Xlffi, 203-204, 1966; xnffi, 99-105, 1968; XIVB, 63, 129, 139, 1971; Space Sci. Rev. 12, 136-186, 1971. Because at the recent General Assemblies some small changes, or corrections, were made, the complete list of Lunar and Martian Topographic Features is published here. Table 1 Lunar Craters Abbe 58S,174E Balboa 19N,83W Abbot 6N,55E Baldet 54S, 151W Abel 34S,85E Balmer 20S,70E Abul Wafa 2N,ll7E Banachiewicz 5N,80E Adams 32S,69E Banting 26N,16E Aitken 17S,173E Barbier 248, 158E AI-Biruni 18N,93E Barnard 30S,86E Alden 24S, lllE Barringer 29S,151W Aldrin I.4N,22.1E Bartels 24N,90W Alekhin 68S,131W Becquerei -

Board Certified Fellows
AMERICAN BOARD OF MEDICOLEGAL DEATH INVESTIGATORS Certificant Directory As of September 30, 2021 BOARD CERTIFIED FELLOWS Addison, Krysten Leigh (Inactive) BC2286 Allmon, James L. BC855 Travis County Medical Examiner's Office Sangamon County Coroner's Office 1213 Sabine Street 200 South 9th, Room 203 PO Box 1748 Springfield, IL 62701 Austin, TX 78767 Amini, Navid BC2281 Appleberry, Sherronda BC1721 Olmsted Medical Examiner's Office Adams and Broomfield County Office of the Coroner 200 1st Street Southwest 330 North 19th Avenue Rochester, MN 55905 Brighton, CO 80601 Applegate, MD, David T. BC1829 Archer, Meredith D. BC1036 Union County Coroner's Office Mohave County Medical Examiner 128 South Main Street 1145 Aviation Drive Unit A Marysville, OH 43040 Lake Havasu, AZ 86404 Bailey, Ted E. (Inactive) BC229 Bailey, Sanisha Renee BC1754 Gwinnett County Medical Examiner's Office Virginia Office of the Chief Medical Examiner 320 Hurricane Shoals Road, NE Central District Lawrenceville, GA 30046 400 East Jackson Street Richmond, VA 23219 Balacki, Alexander J BC1513 Banks, Elsie-Kay BC3039 Montgomery County Coroner's Office Maine Office of the Chief Medical Examiner 1430 Dekalb Street 30 Hospital Street PO Box 311 Augusta, ME 04333 Norristown, PA 19404 Bautista, Ian BC2185 Bayer, Lindsey A. BC875 New York City Office of Chief Medical Examiner District 5 and 24 Medical Examiner Office 421 East 26th Street 809 Pine Street New York, NY 10016 Leesburg, FL 34756 Beck, Shari L BC327 Beckham, Phinon Phillips BC2305 Sedgwick Co Reg. Forensic Science Center Virginia Office of the Chief Medical Examiner 1109 N. Minneapolis Northern District Wichita, KS 67214 10850 Pyramid Place, Suite 121 Manassas, VA 20110 Bednar Keefe, Gale M. -

Carnegie Institution of Washington Monograph Series
BTILL UMI Carnegie Institution of Washington Monograph Series BT ILL UMI 1 The Carnegie Institution of Washington, D. C. 1902. Octavo, 16 pp. 2 The Carnegie Institution of Washington, D. C. Articles of Incorporation, Deed of Trust, etc. 1902. Octavo, 15 pp. 3 The Carnegie Institution of Washington, D. C. Proceedings of the Board of Trustees, January, 1902. 1902. Octavo, 15 pp. 4 CONARD, HENRY S. The Waterlilies: A Monograph of the Genus Nymphaea. 1905. Quarto, [1] + xiii + 279 pp., 30 pls., 82 figs. 5 BURNHAM, S. W. A General Catalogue of Double Stars within 121° of the North Pole. 1906. Quarto. Part I. The Catalogue. pp. [2] + lv + 1–256r. Part II. Notes to the Catalogue. pp. viii + 257–1086. 6 COVILLE, FREDERICK VERNON, and DANIEL TREMBLY MACDOUGAL. Desert Botani- cal Laboratory of the Carnegie Institution. 1903. Octavo, vi + 58 pp., 29 pls., 4 figs. 7 RICHARDS, THEODORE WILLIAM, and WILFRED NEWSOME STULL. New Method for Determining Compressibility. 1903. Octavo, 45 pp., 5 figs. 8 FARLOW, WILLIAM G. Bibliographical Index of North American Fungi. Vol. 1, Part 1. Abrothallus to Badhamia. 1905. Octavo, xxxv + 312 pp. 9 HILL, GEORGE WILLIAM, The Collected Mathematical Works of. Quarto. Vol. I. With introduction by H. POINCARÉ. 1905. xix + 363 pp. +errata, frontispiece. Vol. II. 1906. vii + 339 pp. + errata. Vol. III. 1906. iv + 577 pp. Vol. IV. 1907. vi + 460 pp. 10 NEWCOMB, SIMON. On the Position of the Galactic and Other Principal Planes toward Which the Stars Tend to Crowd. (Contributions to Stellar Statistics, First Paper.) 1904. Quarto, ii + 32 pp. -

History of Wiite Pine Forest Service Blister Rust Control FS-355 a Personal Account HISTORY of WHITE PINE BLISTER RUST CONTROL a PERSONAL ACCOUNT Warren V
United States :5, Department of Agriculture History of WIite Pine Forest Service Blister Rust Control FS-355 A Personal Account HISTORY OF WHITE PINE BLISTER RUST CONTROL A PERSONAL ACCOUNT Warren V. Benedict Former Director Division of Forest Pest Control Forest Service U.S. Department of Agriculture Forest Service Washington, D.C. March 1981 1'or sale by the Su,erintiideiit of Documents, U.S. Government Printing Office Washington, D.C. 20402 Foreword With much personal satisfaction I write this introduc- tion to Warren Benedict's story of white pine blister rust control. I first came in contact with this work in the 1930's. Whenever I visited Blister Rust Control (BRC) camps I always came away with the feeling that these young men were helping to save a valuable natural resource and that in the process they were learning much about forest conserva- tion. Moreover, thousands and thousands of young people were having this useful experience. Several years ago I repeatedly urged that someone write thehistoryoftheBlisterRust Control Program. Obviously, if a history of this kind were done, it should be done by someone who has had actual experience in the pro- gram. If some future historian without such experience should attempt to write the story merely by compiling scat- tered statistics, it would be not only a dry as dust account but the statistics might not be fully understood. Nearly all the former top administrators of the BRC program are gone. As one of the very few top BRC people still alive, we thought that Benedict's nearly half a century of intimate knowledge of this program should be utilized before it was too late. -

Summary of Sexual Abuse Claims in Chapter 11 Cases of Boy Scouts of America
Summary of Sexual Abuse Claims in Chapter 11 Cases of Boy Scouts of America There are approximately 101,135sexual abuse claims filed. Of those claims, the Tort Claimants’ Committee estimates that there are approximately 83,807 unique claims if the amended and superseded and multiple claims filed on account of the same survivor are removed. The summary of sexual abuse claims below uses the set of 83,807 of claim for purposes of claims summary below.1 The Tort Claimants’ Committee has broken down the sexual abuse claims in various categories for the purpose of disclosing where and when the sexual abuse claims arose and the identity of certain of the parties that are implicated in the alleged sexual abuse. Attached hereto as Exhibit 1 is a chart that shows the sexual abuse claims broken down by the year in which they first arose. Please note that there approximately 10,500 claims did not provide a date for when the sexual abuse occurred. As a result, those claims have not been assigned a year in which the abuse first arose. Attached hereto as Exhibit 2 is a chart that shows the claims broken down by the state or jurisdiction in which they arose. Please note there are approximately 7,186 claims that did not provide a location of abuse. Those claims are reflected by YY or ZZ in the codes used to identify the applicable state or jurisdiction. Those claims have not been assigned a state or other jurisdiction. Attached hereto as Exhibit 3 is a chart that shows the claims broken down by the Local Council implicated in the sexual abuse. -
Installers.Pdf
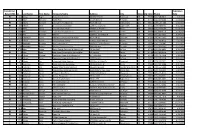
Initial (I) or Expiration Recert (RI) Id Last Name First Sept Name Company Name Address City State Zip Code Phone Date RI 212 Abbas Roger Abbas Construction 6827 NE 33rd St Redmond OR 97756 (541) 548-6812 2/3/2024 I 2888 Adair Benjamin Septic Pros 1751 N Main st Prineville OR 97754 (541) 359-8466 5/10/2024 RI 727 Adams Kenneth Ken Adams Plumbing Inc 906 N 9th Ave Walla Walla WA 99362 (509) 529-7389 6/23/2023 RI 884 Adcock Dean Tri County Construction 22987 SE Dowty Rd Eagle Creek OR 97022 (503) 948-0491 9/25/2023 I 2592 Adelt Georg Cascade Valley Septic 17360 Star Thistle Ln Bend OR 97703 (541) 337-4145 11/18/2022 I 2994 Agee Jetamiah Leavn Trax Excavation LLC 50020 Collar Dr. La Pine OR 97739 (541) 640-3115 9/13/2024 I 2502 Aho Brennon BRX Inc 33887 SE Columbus St Albany OR 97322 (541) 953-7375 6/24/2022 I 2392 Albertson Adam Albertson Construction & Repair 907 N 6th St Lakeview OR 97630 (541) 417-1025 12/3/2021 RI 831 Albion Justin Poe's Backhoe Service 6590 SE Seven Mile Ln Albany OR 97322 (541) 401-8752 7/25/2022 I 2732 Alexander Brandon Blux Excavation LLC 10150 SE Golden Eagle Dr Prineville OR 97754 (541) 233-9682 9/21/2023 RI 144 Alexander Robert Red Hat Construction, Inc. 560 SW B Ave Corvallis OR 97333 (541) 753-2902 2/17/2024 I 2733 Allen Justan South County Concrete & Asphalt, LLC PO Box 2487 La Pine OR 97739 (541) 536-4624 9/21/2023 RI 74 Alley Dale Back Street Construction Corporation PO Box 350 Culver OR 97734 (541) 480-0516 2/17/2024 I 2449 Allison Michael Deschutes Property Solultions, LLC 15751 Park Dr La Pine OR 97739 (541) 241-4298 5/20/2022 I 2448 Allison Tisha Deschutes Property Solultions, LLC 15751 Park Dr La Pine OR 97739 (541) 241-4298 5/20/2022 RI 679 Alman Jay Integrated Water Services 4001 North Valley Dr. -

Curriculum Vitae: Steven L
1 (Revised 06/22/2020) CURRICULUM VITAE: STEVEN L. FORMAN Dept. of Geosciences Baylor University One Bear Place #97354 Waco, Texas 76798 Phone: 254-710-2495, Fax: 254-710-2673 E-mail: [email protected] Web page: http://www.baylor.edu/geology/ PERSONAL DETAILS Born: July 26, 1958; Detroit, Michigan; U.S.A. Citizen Children: Jacob Schneider Forman (9/23/88) & Emma Schneider Forman (2/3/94) EDUCATION B.Sc. in Geology (with distinction), University of Illinois-Urbana, Illinois, May 1981. Senior Honors Thesis: Quaternary till, loess, and soil stratigraphy Athens North Quarry, Menard County, Illinois. Ph.D. in Geological Sciences, University of Colorado, Boulder, December 1986. Dissertation: Quaternary glacial, marine, and soil-developmental history of the Forlandsund area, Spitsbergen, Svalbard. CAREER 2014 to present: Professor of Geosciences/Paleoclimatology, Dept. of Geosciences, Baylor University, Waco, Texas and Director of Geoluminescence Dating Research Laboratory. 1999 to 2014: Professor, Department of Earth and Environmental Sciences, University of Illinois at Chicago, Director, Luminescence Dating Research Laboratory. 1999 to 2001: Acting & founding Director of the Institute of Environmental Science and Policy, University of Illinois at Chicago. 1996 to 1999: Associate Professor (tenured), Department of Earth and Environmental Sciences, University of Illinois at Chicago. 1991 to 1996: Research Scientist, Byrd Polar Research Center, The Ohio State University Columbus, Ohio. 1991 to 1996: Adjunct Assistant Professor, Dept. of Geological Sciences, The Ohio State University, Columbus, Ohio. 1988 to 1991: Assistant Professor (attend. rank), Dept. of Geological Sciences, Univ. of Colorado, Boulder. 1986 to 1991: Fellow and Director of Thermoluminescence Dating Research Laboratory, Center for Geochronological Research, Institute of Arctic & Alpine Research, Univ. -

This Report Runs Every Morning at 0400. All Data Is Current to That Time
Arizona State Forestry Last 24 hr ADC Activity This report runs every morning at 0400. All data is current to that time. Incident2 Trans Date Req Number Req Catalog Item Name Trans Type Resource Mob Plan? Name/Department OR-RSF-000354 KLONDIKE 10/31/2018 O-9460 FACILITIES UNIT LEADER Fill #N/A OR-RSF-000354 KLONDIKE 10/31/2018 O-9460 FACILITIES UNIT LEADER Unfill #N/A OR-RSF-000354 KLONDIKE 10/31/2018 O-9460 FACILITIES UNIT LEADER Unfill #N/A AZ-A3S-001763 SHARPSHOOTER 11/3/2018 E-4 Engine, Type 6 Fill ENGINE - T6X - AZ-FRY - LIC# G-519ED (4X4) (AZ- ADC) AZ-A3S AZ-A3S-001763 SHARPSHOOTER 11/3/2018 E-4.1 ENGINE BOSS Fill KEAN, MIKE AZ-FRY AZ-A5S-001767 ERVING 11/4/2018 E-3 Tender, Water (Support), Type 2 Fill WATER TENDER - TACTICAL - T1 - AZ-PNP - LIC# G- 309HD (WATER TENDER - SUPPORT - T1 - AZ-PNP - LIC# G-309HD) (AZ-ADC) AZ-A5S-001767 ERVING 11/4/2018 E-1 Engine, Type 6 Fill ENGINE - T6X - AZ-PNP - LIC# G-470EH (4X4 - CAFS - FOAM) (AZ-ADC) AZ-A5S-001767 ERVING 11/4/2018 E-2 Engine, Type 6 Fill ENGINE - T6X - AZ-PNP - LIC# G-485GD (4X4 - CAFS - FOAM) (AZ-ADC) Last updated: 11/5/2018 Page 1 of Arizona State Forestry YTD ADC Activity Incident Number/Name Trans Date Req Number Catalogue Name Trans Type Resource Name/Department Mob Plan (MP)? AZ-A5S-001767 ERVING 11/4/18 E-2 Engine, Type 6 Fill ENGINE - T6X - AZ-PNP - LIC# G-485GD (4X4 - CAFS - FOAM) (AZ-ADC) AZ-A5S-001767 ERVING 11/4/18 15:26 E-3 Tender, Water (Support), Type 2 Fill WATER TENDER - TACTICAL - T1 - AZ-PNP - LIC# G-309HD (WATER TENDER - SUPPORT - T1 - AZ-PNP - LIC# G-309HD)