Primary Production and Physiological State of Phytoplankton at the Ushant Tidal Front (West Coast of Brittany, France)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PUB. 143 Sailing Directions (Enroute)
PUB. 143 SAILING DIRECTIONS (ENROUTE) ★ WEST COAST OF EUROPE AND NORTHWEST AFRICA ★ Prepared and published by the NATIONAL GEOSPATIAL-INTELLIGENCE AGENCY Springfield, Virginia © COPYRIGHT 2014 BY THE UNITED STATES GOVERNMENT NO COPYRIGHT CLAIMED UNDER TITLE 17 U.S.C. 2014 FIFTEENTH EDITION For sale by the Superintendent of Documents, U.S. Government Printing Office Internet: http://bookstore.gpo.gov Phone: toll free (866) 512-1800; DC area (202) 512-1800 Fax: (202) 512-2250 Mail Stop: SSOP, Washington, DC 20402-0001 II Preface date of the publication shown above. Important information to amend material in the publication is updated as needed and 0.0 Pub. 143, Sailing Directions (Enroute) West Coast of Europe available as a downloadable corrected publication from the and Northwest Africa, Fifteenth Edition, 2014 is issued for use NGA Maritime Domain web site. in conjunction with Pub. 140, Sailing Directions (Planning Guide) North Atlantic Ocean and Adjacent Seas. Companion 0.0NGA Maritime Domain Website volumes are Pubs. 141, 142, 145, 146, 147, and 148. http://msi.nga.mil/NGAPortal/MSI.portal 0.0 Digital Nautical Charts 1 and 8 provide electronic chart 0.0 coverage for the area covered by this publication. 0.0 Courses.—Courses are true, and are expressed in the same 0.0 This publication has been corrected to 4 October 2014, manner as bearings. The directives “steer” and “make good” a including Notice to Mariners No. 40 of 2014. Subsequent course mean, without exception, to proceed from a point of or- updates have corrected this publication to 24 September 2016, igin along a track having the identical meridianal angle as the including Notice to Mariners No. -

Société Des Régates De Douarnenez, Europe Championship Application
Société des Régates de Douarnenez, Europe championship Application Douarnenez, an ancient fishing harbor in Brittany, a prime destination for water sports lovers , the land of the island, “Douar An Enez” in Breton language The city with three harbors. Enjoy the unique atmosphere of its busy quays, wander about its narrows streets lined with ancient fishermen’s houses and artists workshops. Succumb to the charm of the Plomarch walk, the site of an ancient Roman settlement, visit the Museum Harbor, explore the Tristan island that gave the city its name and centuries ago was the lair of the infamous bandit Fontenelle, go for a swim at the Plage des Dames, “the ladies’ beach”, a stone throw from the city center. The Iroise marine park, a protected marine environment The Iroise marine park is the first designated protected marine park in France. It covers an area of 3500 km2, between the Islands of Sein and Ushant (Ouessant), and the national maritime boundary. Wildlife from seals and whales to rare seabirds can be observed in the park. The Société des Régates de Douarnenez, 136 years of passion for sail. The Société des Régates de Douarnenez was founded in 1883, and from the start competitive sailing has been a major focus for the club. Over many years it has built a strong expertise in the organization of major national and international events across all sailing series. sr douarnenez, a club with five dynamic poles. dragon dinghy sailing kiteboard windsurf classic yachting Discovering, sailing, racing Laser and Optimist one Practicing and promoting Sailing and promoting the Preserving and sailing the Dragon. -

Press Pack Bretagne
2021 PRESS PACK BRETAGNE brittanytourism.com | CONTENTS Editorial p. 03 Slow down Take your foot off the pedal p. 04 Live Experience the here and now p. 12 Meet Immerse yourself in local culture p. 18 Share Together. That’s all ! p. 24 Practical information p. 29 EDITORIAL Bon voyage 2020 has been an unsettling and air and the wide open spaces. Embracing with the unexpected. A concentration of challenging year and we know that many the sea. Enjoying time with loved ones. the essential, and that’s why we love it! It of you have had to postpone your trip to Trying something new. Giving meaning to invites you to slow down, to enjoy life, to Brittany. These unusual times have been our daily lives. Meeting passionate locals. meet and to share: it delivers its promise a true emotional rollercoaster. They Sharing moments and laughs. Creating of uniqueness. So set sail, go for it, relax have also sharpened our awareness and memories for the years to come. and enjoy it as it comes. Let the eyes do redesigned our desires. We’re holding on to Let’s go for an outing to the Breton the talking and design your next holiday our loved ones, and the positive thoughts peninsula. A close, reassuring destination using these pages. of a brighter future. We appreciate even just across the Channel that we think we We wish you a wonderful trip. more the simple good things in life and know, but one that takes us far away, on the importance of seizing the moment. -

Molène Island
> MOLÈNE IN A FEW QUESTIONS... > PREPARE YOUR STAY • Prientit ho peaj > SHOPS AND RESTAURANTS • Stalioù-kenwerzh ha pretioù • Molenez gant un nebeud goulennoù www.pays-iroise.bzh and www.molene.fr Shops How to get here - General food store: 02 98 07 38 81 How does the island get its water supply? - Tobacconist’s, newspapers, souvenirs: 02 98 07 39 71 The island is now self-sufficient in its water management, thanks to two collective rainwater Departures all year from the following harbour stations: - Atelier des algues (seaweed workshop): 02 98 07 39 71 recovery supplies: the English water tank offered by Queen Victoria in 1896 and the - departures from Brest and Le Conquet by passenger ferry: 02 98 80 80 80 / www.pennarbed.fr - Les jardins de la Chimère (creation of jewellery): 02 98 07 37 32 impluvium built in 1976. We can also count 3 water catchments (ground water) located - departures from Le Conquet by individual taxi boats: 06 71 88 74 21 northwest of the island and private supplies, which are beginning to appear in many homes. During peak season from the following harbour stations: Warning: there is no ATM on the island. - departures from Camaret: 02 98 27 88 22 / www.pennarbed.fr Direct sale of fish and shellfish by the professional fishermen of Molène Is there a school on the island? - departures from Camaret, Lanildut and Le Conquet: 08 25 13 52 35 / www.finist-mer.fr - Berthelé Philippe (shellfish only): 06 32 71 26 75 From primary to secondary school, the children of Molène go to school on the island. -

Commissioned Report
COMMISSIONED REPORT Annex to Commissioned Report No. 271 A review of relevant experience of coastal and marine national parks Case studies (1 & 2) Parc Naturel Régional d’Armorique & Parc Naturel Marin d’Iroise - Brittany, France Prepared by Sue Wells for Hambrey Consulting This report should be cited as: Wells, S. (2008). Case studies 1 & 2 - Parc Naturel Régional d’Armorique and Parc Naturel Marin d’Iroise, Brittany, France. Scottish Natural Heritage Research, Annex to Commissioned Report No. 271 - Available online at http://www.snh.org.uk/strategy/CMNP/sr-adnp01.asp This report is one of a number of studies undertaken to inform the following publication: Hambrey Consulting (2008). A review of relevant experience of coastal and marine national parks. Scottish Natural Heritage Commissioned Report No. 271 (ROAME No. R07NC). This report, or any part of it, should not be reproduced without the permission of Scottish Natural Heritage. This permission will not be withheld unreasonably. The views expressed by the author(s) of this report should not be taken as the views and policies of Scottish Natural Heritage. © Scottish Natural Heritage 2008 Sketch profiles Parc Naturel Régional d’Armorique (PNRA) Location and size Includes four geographically defined areas of Finistere, northern Brittany: Ouessant-Molene archipelago (Ushant Islands); Crozon peninsula; Monts d’Arree; and estuary of the River Aulne. Total area 172,000 ha. Scope Primarily terrestrial, but includes 60,000 ha of marine water to 30 m depth (the marine area also falls within the proposed Parc Naturel Marin d’Iroise); other protected areas lie within its boundaries including the Reserve Biosphere d’Iroise and several terrestrial nature reserves. -

Sea & Shore Technical Newsletter
1 CENTRE OF DOCUMENTATION, RESEARCH AND EXPERIMENTATION ON ACCIDENTAL WATER POLLUTION 715, Rue Alain Colas, CS 41836 - 29218 BREST CEDEX 2, FRANCE Tel: +33 (0)2 98 33 10 10 – Fax: +33 (0)2 98 44 91 38 Email: [email protected] - Web: www.cedre.fr Sea & Shore Technical Newsletter n°40 22001144---22 Contents • Spills ................................................................................................................................................. 2 Pollution in a port following failure of the dredger Prins IV (Port-Diélette, Manche, France) ............................ 2 Heavy fuel oil spill in a protected mangrove: collision of the Southern Star 7 (Bangladesh) ............................ 2 Heavy fuel oil spill in a port: the bulk carrier Lord Star (Brest, Brittany, France) ............................................... 5 Landslide and damage to the Tikhoretsk-Tuapse-2 pipeline (Black Sea, Russia) ............................................ 6 • Past spills ........................................................................................................................................ 7 Deepwater Horizon spill: BP agrees to pay out record-breaking compensation ............................................... 7 Environmental damages caused by the Exxon Valdez spill: an end to legal action for the US authorities ....... 7 • Review of spills having occurred worldwide in 2014 .................................................................. 7 Oil and HNS spills, all origins (Cedre analysis) ............................................................................................... -

With DOL4DE4BRETAGNE 02 99 48 15 37 PONT4CROIX 02 98 70 40 38
Tourist information centres SB Sensation Bretagne Want to get back to Want to hunt out some of Brittany and Loire Atlantique SV Station Verte Bretagne Calling from abroad, add +33 before the number without “0” Nature ? heritage? Côtes d’Armor BREST 02 98 44 24 96 BRIEC4DE4L’ODET 02 98 57 74 62 BELLE4ISLE4EN4TERRE 02 96 43 01 71 BRIGNOGAN 02 98 83 41 08 Let the sea air recharge your batteries! Plunge right in and discover the medieval cities BINIC SB 02 96 73 60 12 CAMARET4SUR4MER 02 98 27 93 60 Discover the rich variety of landscapes in Brittany. Land of the sea Brittany’s historic past is best hunted on foot. You’ll fi nd it lurking BRÉHAT ÎLE 02 96 20 04 15 CARANTEC SB 02 98 67 00 43 (‘Armor’), Brittany has 2,700 km of coastal scenery: in fact, a third down a spiral stone staircase, quietly gleaming in a square CALLAC SV 02 96 45 59 34 CARHAIX4PLOUGUER 02 98 93 04 42 of the French coastline is in Brittany! You’ll love the constantly surrounded by half-timbered houses, or tucked away behind a gap CHÂTELAUDREN 02 96 79 77 71 CHÂTEAULIN 02 98 86 02 11 changing views, sometimes up close and personal, sometimes in the city walls. As you stroll the little back streets in Brittany’s many DINAN 02 96 87 69 76 CHATEAUNEUF4DU4FAOU 02 98 81 83 90 distant and majestic: cliff s, coves, dunes, sandy beaches – Brittany ‘Small Towns of Character’ (‘Petites Cités de Caractères’) and ‘Towns ERQUY SB 02 96 72 30 12 CLÉDER 02 98 69 43 01 ÉTABLES4SUR4MER 02 96 70 65 41 has it all. -

Piscine Chauffée À Deux Pas De La Plage
Piscine chauffée à deux pas de la plage L o ca tio n d’e mp lac emen ts et de mo bil- hom es Plouguerneau / Finistère / Bretagne Entrez dans l’histoire et la tradition La côte sauvage de Bretagne vous offre de magnifiques paysages, avec ses plages, ses rochers et ses ports, la Pointe du Raz, l’îlot fortifié de Bertheaume, les îles d’Ouessant, Molène... Venez en découvrir davantage : - Le musée des Goémoniers et de l’algue à Plouguerneau, - Le village des Pêcheurs (Ménéham) à Kerlouan, - Le phare de l’Ile Vierge, - À Brest, Océanopolis, le port de plaisance, l’arsenal avec son musée... - Dégustation des spécialités bretonne (Kouign Amann, Kig Ha Farz, crustacés...) The wild coast of Brittany offers beautiful landscapes, with its sand beaches, its boulders and its harbours, the Pointe du Raz, the fortified island of Bertheaume, the Ushant islands, Molène… Come and discover more about it : - The Plouguerneau’s eco-museum about seaweed harvesters, - The fishing village (Ménéham) of Kerlouan, - The Île Vierge’s lighthouse, - In Brest, Oceanopolis, the marina, the arsenal with its museum… - Tasting of Breton specialities (Kouign Amann, Kig Ha Farz, crustaceans...) oûter a Venez g ux plaisirs de l’eau ! SIN S A B É F F C H A U Loisirs sur le camping enez goûter aux plais V irs de l’eau ! Le temps d’une longueur ou d’un plongeon, venez profiter de notre piscine couverte et chauffée. Petits et grands apprécieront ce moment de détente et d’amusement. Les plus petits trouveront leur bonheur dans la pataugeoire où ils pourront s’épanouir pendant que les plus grands pourront se détendre dans le bassin ! Un coin balnéo vous attend. -
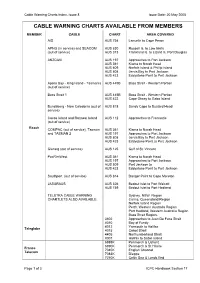
Cable Warning Chart Coverage
Cable Warning Charts Index, Issue 8 Issue Date: 20 May 2005 CABLE WARNING CHARTS AVAILABLE FROM MEMBERS MEMBER CABLE CHART AREA COVERED AIS AUS 754 Lancelin to Cape Peron APNG (in service) and SEACOM AUS 830 Russell Is. to Low Islets (out of service) AUS 373 Frankland Is. to Lizard Is, Port Douglas ANZCAN AUS 197 Approaches to Port Jackson AUS 361 Kiama to Norah Head AUS 609 Norfolk Island & Phillip Island AUS 808 Jervis Bay to Port Jackson AUS 423 Eddystone Point to Port Jackson Apollo Bay - King Island - Tasmania AUS 445B Bass Strait - Western Portion (out of service) Bass Strait 1 AUS 445B Bass Strait - Western Portion AUS 422 Cape Otway to Gabo Island Bundaberg - New Caledonia (out of AUS 818 Sandy Cape to Bustard Head service) Cocos Island and Rottnest Island AUS 112 Approaches to Fremantle (out of service) Reach COMPAC (out of service), Tasman AUS 361 Kiama to Norah Head and TASMAN 2 AUS 197 Approaches to Port Jackson AUS 808 Jervis Bay to Port Jackson AUS 423 Eddystone Point to Port Jackson Glenelg (out of service) AUS 125 Gulf of St. Vincent PacRimWest AUS 361 Kiama to Norah Head AUS 197 Approaches to Port Jackson AUS 809 Port Jackson to AUS 423 Eddystone Point to Port Jackson Southport (out of service) AUS 814 Danger Point to Cape Moreton JASURAUS AUS 326 Bedout Islet to Port Walcott AUS 739 Bedout Islet to Port Hedland TELSTRA CABLE WARNING Sydney, NSW Region CHARTLETS ALSO AVAILABLE Cairns, Queensland Region Norfolk Island Region Perth, Western Australia Region Port Hedland, Western Australia Region Bass Strait Region. -

This Work Is Protected by Copyright and Other Intellectual Property Rights
This work is protected by copyright and other intellectual property rights and duplication or sale of all or part is not permitted, except that material may be duplicated by you for research, private study, criticism/review or educational purposes. Electronic or print copies are for your own personal, non- commercial use and shall not be passed to any other individual. No quotation may be published without proper acknowledgement. For any other use, or to quote extensively from the work, permission must be obtained from the copyright holder/s. POLYOROGENIC EVOLUTION OF THE WESTERN PAYS DE LEON. FINISTERE. FRANCE H. ROPER PH.D. 1980 VOL.1: TEXT , BEST COpy . , .AVAILABLE . Variable print quality ABSTRACT A completely new 11thological classification is made of the Lannilis Metamorphic Complex and the Plouguerneau Mi~atites, which lie south and North respectively of the Porspoder Lineament in the north-west Pays de Leon. The sequence of metamorphic, magmatic and tectonic episodes is analysed and a correlation between the two complexes is suggested. The metasedimentary Iithologies are thought to be derived from Brioverian semi-peli tes, and the meta-igneous rocks from post-Brioverian intrusions. The later granitic rocks associated with the Plouguerneau Migmatites are described and classified in detail for the first time. The structures which define the Porspoder Lineament itself are also described. This feature may have originally been an intra-continental fault of transform type. Existing geochronological data and new K-Ar mineral dates are used to fit the geological evidence from the NW Pays de Leon to the absolute time scale. The importance of the Hercynian orogeny is emphasised; the main metamorphism and migmatisation are attributed to the Bretonic (Devono-Carboniferous) phase. -

KISH Summer 2009 ICC
Revisiting Northern Spain Noel Casey Dublin to La Rochelle of Ile de Re to pass under the bridge to arrive at La Rochelle n 2006, during the Irish Cruising Club’s rally to northern (Minimes), mostly sailing some 60 miles each day in ideal ISpain, which visited Hondabria, Zumia and Bilbao, circum - weather. At La Rochelle in late June the temperature was 35 stances dictated that I was unable to arrive in time to the first degrees, the highest experienced in our time away. After a two- two stops on that excellent rally, but having really enjoyed the day stopover, we headed south for Hondabria. closing event in Bilbao, it seemed essential to revisit those This journey generates several interesting navigational missed ports. conundrums, the first being that there is no shelter from the A trip to northern Spain via south Brittany by Kish, my prevailing northwesterlies, making for a very long, 150 mile Tayana 37, commenced on June 14th 2009, leaving Dun lee-shore on the French coast, with only a couple of small Laoghaire in a fresh southeasterly. We then made stopovers in harbours with harbour bars untenable in strong westerlies. Wicklow and Kilmore Quay, where we waited two days for a Secondly there is a military artillery exercise area stretching favorable slant in the fresh winds. A projected stopover in the from five miles to forty-five miles offshore for most of that Scillies was abandoned, and after some 50 hours at sea, John Dunlop and I arrived in Concarneau, having passed to the west of the Chenal du Four and Raz de Sein in the night, the inshore passage being a difficult navigational challenge at night with a south going tide against a southerly wind. -

Oceanographic Structure and Seasonal Variation Contribute To
1 Published in ICES J. Mar. Sci (2021) - https://doi.org/10.1093/icesjms/fsab127 2 Oceanographic structure and seasonal variation 3 contribute to high heterogeneity in mesozooplankton 4 over small spatial scales 5 6 Manoela C. Brandão1*, Thierry Comtet2, Patrick Pouline3, Caroline Cailliau3, Aline 7 Blanchet-Aurigny1, Marc Sourisseau4, Raffaele Siano4, Laurent Memery5, Frédérique 8 Viard6, and Flavia Nunes1* 9 10 1Ifremer Centre de Bretagne, DYNECO, Laboratory of Coastal Benthic Ecology, Plouzané, France 11 2Sorbonne Université, CNRS, UMR 7144 AD2M, Station Biologique de Roscoff, Place G. Teissier, 12 Roscoff, France 13 3Office Français de la Biodiversité, Parc Naturel Marin d'Iroise, Le Conquet, France 14 4Ifremer Centre de Bretagne, DYNECO, PELAGOS, Plouzané, France 15 5Laboratoire des Sciences de l'Environnement Marin (LEMAR), UMR CNRS/IFREMER/IRD/UBO 6539, 16 Plouzané, France 17 6ISEM, Univ Montpellier, CNRS, EPHE, IRD, Montpellier, France 18 *Corresponding authors: [email protected] (MCB), [email protected] (FN) 19 20 Abstract 21 The coastal oceans can be highly variable, especially near ocean fronts. The Ushant Front is the 22 dominant oceanographic feature in the Iroise Sea (NE Atlantic) during summer, separating warm 23 stratified offshore waters from cool vertically-mixed nearshore waters. Mesozooplankton 24 community structure was investigated over an annual cycle to examine relationships with 25 oceanographic conditions. DNA metabarcoding of COI and 18S genes was used in communities 26 from six sites along two cross-shelf transects. Taxonomic assignments of 380 and 296 OTUs (COI 27 and 18S respectively) identified 21 classes across 13 phyla. Meroplankton relative abundances 28 peaked in spring and summer, particularly for polychaete and decapod larvae respectively, 29 corresponding to the reproductive periods of these taxa.