Spatial Genetic Structure and Demographic Inference of the Patagonian Squid Doryteuthis Gahi in the South-Eastern Pacific Ocean Christian M
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Impacts of Climate Change on Marine Fisheries and Aquaculture in Chile
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/319999645 Impacts of Climate Change on Marine Fisheries and Aquaculture in Chile Chapter · September 2017 DOI: 10.1002/9781119154051.ch10 CITATIONS READS 0 332 28 authors, including: Nelson A Lagos Ricardo Norambuena University Santo Tomás (Chile) University of Concepción 65 PUBLICATIONS 1,052 CITATIONS 13 PUBLICATIONS 252 CITATIONS SEE PROFILE SEE PROFILE Claudio Silva Marco A Lardies Pontificia Universidad Católica de Valparaíso Universidad Adolfo Ibáñez 54 PUBLICATIONS 432 CITATIONS 70 PUBLICATIONS 1,581 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Irish moss - green crab interactions View project Influence of environment on fish stock assessment View project All content following this page was uploaded by Pedro A. Quijón on 11 November 2017. The user has requested enhancement of the downloaded file. 239 10 Impacts of Climate Change on Marine Fisheries and Aquaculture in Chile Eleuterio Yáñez1, Nelson A. Lagos2,13, Ricardo Norambuena3, Claudio Silva1, Jaime Letelier4, Karl-Peter Muck5, Gustavo San Martin6, Samanta Benítez2,13, Bernardo R. Broitman7,13, Heraldo Contreras8, Cristian Duarte9,13, Stefan Gelcich10,13, Fabio A. Labra2, Marco A. Lardies11,13, Patricio H. Manríquez7, Pedro A. Quijón12, Laura Ramajo2,11, Exequiel González1, Renato Molina14, Allan Gómez1, Luis Soto15, Aldo Montecino16, María Ángela Barbieri17, Francisco Plaza18, Felipe Sánchez18, -

Concholepas Concholepas
ANNALS OF THE SOUTH AFRICAN MUSEUM ANNALE VAN DIE SUID-AFRIKAANSE MUSEUM Volume 97 Band October 1985 Oktober Part 1 Deel THE FOSSIL OCCURRENCE IN SOUTHERN AFRICA OF THE SOUTH AMERICAN INTERTIDAL MOLLUSC CONCHOLEPAS CONCHOLEPAS By BRIAN KENSLEY are issued in parts at irregular intervals as material becomes available word uitgegee in dele op ongereelde tye na gelang van die beskikbaarheid van stof 1,2(1-3,5-8),3(1-2,4-5,8, I.-p.i.), 5(1-3, 5, 7-9), 6(1, I.-p.i.), 7(1-4), 8, 9(1-2, 7), 10(1-3), 11(1-2,5,7, I.-p.i.), 14(1-2), 15(4-5),24(2),27,31(1-3),32(5),33,36(2),45(1) Printed in South Africa by In Suid-Afrika gedruk deur The Rustica Press, Pty., Ltd., Die Rustica-pers, Edms., Bpk., Court Road, Wynberg, Cape Courtweg, Wynberg, Kaap THE FOSSIL OCCURRENCE IN SOUTHERN AFRICA OF THE SOUTH AMERICAN INTERTIDAL MOLLUSC CONCHOLEPASCONCHOLEPAS BRIAN KENSLEY National Museum of Natural History, Smithsonian Institution, Washington, D. C. The occurrence of the thaidid gastropod genus Concholepas is recorded from presumed Late Pleistocene coastal deposits in southern South West Africa-Namibia. The material is indistinguishable from C. concholepas, a species known from the Pliocene to Recent on the west coast of South America. The living species characteristically occurs in cold-temperate waters from the intertidal to depths of 40 m. It is suggested that the southern African fossils represent a short-lived pioneer population, established by larvae drifting from South America. -
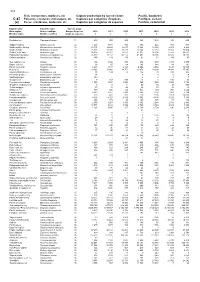
Fish, Crustaceans, Molluscs, Etc Capture Production by Species
534 Fish, crustaceans, molluscs, etc Capture production by species items Pacific, Southeast C-87 Poissons, crustacés, mollusques, etc Captures par catégories d'espèces Pacifique, sud-est (a) Peces, crustáceos, moluscos, etc Capturas por categorías de especies Pacífico, sudoriental English name Scientific name Species group Nom anglais Nom scientifique Groupe d'espèces 2010 2011 2012 2013 2014 2015 2016 Nombre inglés Nombre científico Grupo de especies t t t t t t t Flatfishes nei Pleuronectiformes 31 613 302 806 323 1 804 483 693 Tadpole codling Salilota australis 32 1 400 1 091 768 374 522 703 536 Southern blue whiting Micromesistius australis 32 23 301 19 629 16 675 15 304 10 036 8 809 8 269 Southern hake Merluccius australis 32 25 361 20 909 20 288 19 346 12 393 16 150 16 804 South Pacific hake Merluccius gayi 32 90 305 82 977 72 872 92 031 96 196 77 283 98 662 Patagonian grenadier Macruronus magellanicus 32 74 330 70 137 62 175 47 602 39 138 37 475 28 108 Chilean grenadier Coelorinchus chilensis 32 156 134 136 91 54 59 47 Sea catfishes nei Ariidae 33 406 1 426 852 876 1 217 1 185 1 285 Snake eels nei Ophichthidae 33 38 65 114 142 144 49 181 Pacific cornetfish Fistularia corneta 33 6 443 4 513 4 323 4 854 2 534 7 630 19 559 Mullets nei Mugilidae 33 10 821 13 400 18 751 13 876 14 290 14 044 17 345 Snooks(=Robalos) nei Centropomus spp 33 98 104 78 136 79 310 305 Broomtail grouper Mycteroperca xenarcha 33 14 .. -

(Approx) Mixed Micro Shells (22G Bags) Philippines € 10,00 £8,64 $11,69 Each 22G Bag Provides Hours of Fun; Some Interesting Foraminifera Also Included
Special Price £ US$ Family Genus, species Country Quality Size Remarks w/o Photo Date added Category characteristic (€) (approx) (approx) Mixed micro shells (22g bags) Philippines € 10,00 £8,64 $11,69 Each 22g bag provides hours of fun; some interesting Foraminifera also included. 17/06/21 Mixed micro shells Ischnochitonidae Callistochiton pulchrior Panama F+++ 89mm € 1,80 £1,55 $2,10 21/12/16 Polyplacophora Ischnochitonidae Chaetopleura lurida Panama F+++ 2022mm € 3,00 £2,59 $3,51 Hairy girdles, beautifully preserved. Web 24/12/16 Polyplacophora Ischnochitonidae Ischnochiton textilis South Africa F+++ 30mm+ € 4,00 £3,45 $4,68 30/04/21 Polyplacophora Ischnochitonidae Ischnochiton textilis South Africa F+++ 27.9mm € 2,80 £2,42 $3,27 30/04/21 Polyplacophora Ischnochitonidae Stenoplax limaciformis Panama F+++ 16mm+ € 6,50 £5,61 $7,60 Uncommon. 24/12/16 Polyplacophora Chitonidae Acanthopleura gemmata Philippines F+++ 25mm+ € 2,50 £2,16 $2,92 Hairy margins, beautifully preserved. 04/08/17 Polyplacophora Chitonidae Acanthopleura gemmata Australia F+++ 25mm+ € 2,60 £2,25 $3,04 02/06/18 Polyplacophora Chitonidae Acanthopleura granulata Panama F+++ 41mm+ € 4,00 £3,45 $4,68 West Indian 'fuzzy' chiton. Web 24/12/16 Polyplacophora Chitonidae Acanthopleura granulata Panama F+++ 32mm+ € 3,00 £2,59 $3,51 West Indian 'fuzzy' chiton. 24/12/16 Polyplacophora Chitonidae Chiton tuberculatus Panama F+++ 44mm+ € 5,00 £4,32 $5,85 Caribbean. 24/12/16 Polyplacophora Chitonidae Chiton tuberculatus Panama F++ 35mm € 2,50 £2,16 $2,92 Caribbean. 24/12/16 Polyplacophora Chitonidae Chiton tuberculatus Panama F+++ 29mm+ € 3,00 £2,59 $3,51 Caribbean. -

Hemocyanin of the Molluscan Concholepas Concholepas Exhibits an Unusual Heterodecameric Array of Subunits*
THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 279, No. 25, Issue of June 18, pp. 26134–26142, 2004 © 2004 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. Hemocyanin of the Molluscan Concholepas concholepas Exhibits an Unusual Heterodecameric Array of Subunits* Received for publication, January 28, 2004, and in revised form, April 7, 2004 Published, JBC Papers in Press, April 8, 2004, DOI 10.1074/jbc.M400903200 Pablo De Ioannes‡, Bruno Moltedo‡, Harold Oliva, Rodrigo Pacheco, Fernando Faunes, Alfredo E. De Ioannes, and Marı´a Ine´s Becker§ From the Department of Research and Development, BIOSONDA Corp., Avenida Alcalde Eduardo Castillo Velasco 2902, Santiago 7750269, Chile We describe here the structure of the hemocyanin Molluscan hemocyanins assemble into hollow cylindrical lay- from the Chilean gastropod Concholepas concholepas ered molecules formed by 10 subunits. Each subunit, ranging (CCH), emphasizing some attributes that make it inter- from 350 to 450 kDa, includes seven or eight globular folded esting among molluscan hemocyanins. CCH exhibits a domains known as functional units (FUs),1 which are arranged predominant didecameric structure as revealed by elec- into pearl-like chains and covalently bound by a short flexible tron microscopy and a size of 8 MDa by gel filtration, linker region of 10–15 amino acid residues. FUs vary in size and, in contrast with other mollusc hemocyanins, its from 45 to 55 kDa, and each of them is capable of reversibly 2؉ stabilization does not require additional Ca and/or binding one oxygen molecule through a pair of copper atoms. Downloaded from 2؉ Mg in the medium. -

Genetic Structure and Diversity of Squids with Contrasting Life Histories in the Humboldt Current System Estructura Y Diversidad
Genetic diversity of squids Hidrobiológica 204, 24 (): 1-0 Genetic structure and diversity of squids with contrasting life histories in the Humboldt Current System Estructura y diversidad genética de calamares con historias de vida contrastantes en el Sistema de Corrientes de Humboldt Christian Marcelo Ibáñez and Elie Poulin Instituto de Ecología y Biodiversidad, Departamento de Ciencias Ecológicas, Facultad de Ciencias, Universidad de Chile. Las Palmeras # 3425, Ñuñoa. Chile e-mail: [email protected] Ibáñez C. M and Elie Poulin. 204. Genetic structure and diversity of squids with contrasting life histories in the Humboldt Current System. Hidrobiológica 24 (): -0. ABSTRACT Dosidiscus gigas and Doryteuthis gahi are the most abundant squids in the Humboldt Current System (HCS). These species have contrasting life histories. To determine the genetic structure and diversity of these species, we collected samples from different places in the HCS and amplified a fragment of the mitochondrial cytochrome C oxidase I gene. The molecular analysis of D. gigas revealed low genetic diversity, absence of population structure and evidence for a demographic expansion during the transition from the last glacial period to the current interglacial. These results sug- gest that D. gigas is composed of one large population with high levels of gene flow throughout the HCS. In the case of D. gahi, the sequences indicated the presence of two population units in the HCS, one in south-central Chile and one in Peru. The Chilean unit had greater genetic diversity, suggesting that it is an old, relatively stable population. In the Peruvian unit there was less genetic diversity and evidence of a recent demographic expansion. -

Shelled Molluscs
Encyclopedia of Life Support Systems (EOLSS) Archimer http://www.ifremer.fr/docelec/ ©UNESCO-EOLSS Archive Institutionnelle de l’Ifremer Shelled Molluscs Berthou P.1, Poutiers J.M.2, Goulletquer P.1, Dao J.C.1 1 : Institut Français de Recherche pour l'Exploitation de la Mer, Plouzané, France 2 : Muséum National d’Histoire Naturelle, Paris, France Abstract: Shelled molluscs are comprised of bivalves and gastropods. They are settled mainly on the continental shelf as benthic and sedentary animals due to their heavy protective shell. They can stand a wide range of environmental conditions. They are found in the whole trophic chain and are particle feeders, herbivorous, carnivorous, and predators. Exploited mollusc species are numerous. The main groups of gastropods are the whelks, conchs, abalones, tops, and turbans; and those of bivalve species are oysters, mussels, scallops, and clams. They are mainly used for food, but also for ornamental purposes, in shellcraft industries and jewelery. Consumed species are produced by fisheries and aquaculture, the latter representing 75% of the total 11.4 millions metric tons landed worldwide in 1996. Aquaculture, which mainly concerns bivalves (oysters, scallops, and mussels) relies on the simple techniques of producing juveniles, natural spat collection, and hatchery, and the fact that many species are planktivores. Keywords: bivalves, gastropods, fisheries, aquaculture, biology, fishing gears, management To cite this chapter Berthou P., Poutiers J.M., Goulletquer P., Dao J.C., SHELLED MOLLUSCS, in FISHERIES AND AQUACULTURE, from Encyclopedia of Life Support Systems (EOLSS), Developed under the Auspices of the UNESCO, Eolss Publishers, Oxford ,UK, [http://www.eolss.net] 1 1. -

Os Nomes Galegos Dos Moluscos 2020 2ª Ed
Os nomes galegos dos moluscos 2020 2ª ed. Citación recomendada / Recommended citation: A Chave (20202): Os nomes galegos dos moluscos. Xinzo de Limia (Ourense): A Chave. https://www.achave.ga /wp!content/up oads/achave_osnomesga egosdos"mo uscos"2020.pd# Fotografía: caramuxos riscados (Phorcus lineatus ). Autor: David Vilasís. $sta o%ra est& su'eita a unha licenza Creative Commons de uso a%erto( con reco)ecemento da autor*a e sen o%ra derivada nin usos comerciais. +esumo da licenza: https://creativecommons.org/ icences/%,!nc-nd/-.0/deed.g . Licenza comp eta: https://creativecommons.org/ icences/%,!nc-nd/-.0/ ega code. anguages. 1 Notas introdutorias O que cont!n este documento Neste recurso léxico fornécense denominacións para as especies de moluscos galegos (e) ou europeos, e tamén para algunhas das especies exóticas máis coñecidas (xeralmente no ámbito divulgativo, por causa do seu interese científico ou económico, ou por seren moi comúns noutras áreas xeográficas) ! primeira edición d" Os nomes galegos dos moluscos é do ano #$%& Na segunda edición (2$#$), adicionáronse algunhas especies, asignáronse con maior precisión algunhas das denominacións vernáculas galegas, corrixiuse algunha gralla, rema'uetouse o documento e incorporouse o logo da (have. )n total, achéganse nomes galegos para *$+ especies de moluscos A estrutura )n primeiro lugar preséntase unha clasificación taxonómica 'ue considera as clases, ordes, superfamilias e familias de moluscos !'uí apúntanse, de maneira xeral, os nomes dos moluscos 'ue hai en cada familia ! seguir -
Full Text in Pdf Format
MARINE ECOLOGY PROGRESS SERIES Published July 20 Mar Ecol Prog Ser 1 Effects of human activities on long-term trends in sandy beach populations: the wedge clam Donax hanleyanus in Uruguay Omar Defeo*, Anita de Alava Instituto Nacional de Pesca, Casilla de Correo 1612, 11200 Montevideo, Uruguay ABSTRACT: A long-term analysis of the structure of a bivalve population consisting of the wedge clam Donax hanleyanus is described for an exposed temperate sandy beach of Uruguay. The potential effects of human harvesting on the sympatric bivalve Mesodesma mactroides (yellow clam) and of salinity were analyzed through time and space (longshore variation). Inter- and intra-annual fluctua- tions of the different population components (recruits, juveniles and adults) were detected. D. han- leyanus showed uneven periods of abundance, with the occurrence of peaks of different magnitude that appeared related to fluctuations in the fishing effort targeting on the yellow clam. D. hanlepanus showed a marked longshore variability in population structure and abundance along the 22 km of sandy beach sampled Spatial variations in salinity and also in the amount of fishing effort exerted on Ad. mactroides seem to be key factors in explaining th~svariation. This study suggests that further research on sandy beach populations should include human activities as important factors affecting long-term trends. KEY WORDS: Donax - Bivalves . Sandy beach. Long-term - Human impact. Uruguay INTRODUCTION beaches, which makes up the greatest proportion of most open shores (McLachlan 1990), has usually been Exposed marine beaches are physically stressed the focus of short-termhnstantaneous research, mainly environments (sensu McLachlan 1983, 1988), and the related to comn~unitystructure and zonation (Jaramillo invertebrate populations and communities living there 1978, Donn 1987, McLachlan 1990, Jaramillo & Mc- are usually considered to be regulated mainly by Lachlan 1993).Very little is known about the long-term physical factors. -

Introduction Laevistrombus Canarium (Linnaeus 1758)
Journal of Sustainability Science and Management e-ISSN: 2672-7226 Volume 14 Number 1, February 2019 : 1-10 © Penerbit UMT EFFECTS OF TEMPERATURE ON FOOD CONSUMPTION OF JUVENILES DOG CONCH, Laevistrombus canarium (LINNAEUS, 1758) IN LABORATORY CONDITION WAN NURUL HUSNA WAN HASSAN1*, NURUL AMIN SM2, MAZLAN ABD GHAFFAR1,3,4 AND ZAIDI CHE COB1,3 1School of Environmental and Natural Resources Science, Faculty of Science and Technology, Universiti Kebangsaan Malaysia, 43600 Bangi, Selangor, Malaysia. 2Department of Aquaculture, Faculty of Agriculture, Universiti Putra Malaysia, 43400, Serdang, Selangor, Malaysia. 3Marine Ecosystem Research Centre, Universiti Kebangsaan Malaysia, 43600 Bangi, Selangor, Malaysia. 4Institute of Marine Biotechnology, Universiti Malaysia Terengganu, 21030 Kuala Nerus, Terengganu, Malaysia *Corresponding author: [email protected] Abstract: The dog conch, Laevistrombus canarium (Linnaeus, 1758) is one of the economically important marine molluscs that have high market value, particularly in the Southeast Asian region. This study investigates food consumption and assimilation by the juvenile conch at different temperature regimes (22, 26, 30 and 34°C). Live samples of the juvenile conch were collected on several occasions between December 2013 to May 2105 at Merambong shoal, Johor Straits, Malaysia. They were acclimatized for one week in stocking aquaria with well-aerated seawater at 30 PSU, 26°C and fed with commercial marine sinking pellets. Prior to experimentation, the gastric emptying levels of the samples were standardized by allowing them to feed until satiation, followed by 24 hrs starvation. All treatments were carried out in ten replicates of similar sized aquarium (20 x 15 x 15 cm) containing 4L of aerated seawater. The conch food consumption rate was significantly different (p<0.05) between different temperature regimes. -

Quantification and Distribution of Omega-3 Fatty Acids in South Pacific Fish and Shellfish Species
foods Article Quantification and Distribution of Omega-3 Fatty Acids in South Pacific Fish and Shellfish Species Miguel Ángel Rincón-Cervera 1,*, Valeria González-Barriga 1, Jaime Romero 1 , Rodrigo Rojas 2 and Sandra López-Arana 3 1 Instituto de Nutrición y Tecnología de los Alimentos (INTA), Universidad de Chile, Avda. El Líbano 5524, Macul, Santiago 7830490, Chile; [email protected] (V.G.-B.); [email protected] (J.R.) 2 Departamento de Acuicultura, Facultad de Ciencias del Mar, Universidad Católica del Norte, Larrondo 1281, Coquimbo 1781421, Chile; [email protected] 3 Departamento de Nutrición, Facultad de Medicina, Universidad de Chile, Avda. Independencia 1027, Independencia, Santiago 8380453, Chile; [email protected] * Correspondence: [email protected]; Tel.: +56-2-29781449 Received: 22 January 2020; Accepted: 17 February 2020; Published: 21 February 2020 Abstract: Fatty acid composition and distribution in edible species of fish and shellfish captured in the South Pacific were studied, with a focus on n-3 long-chain polyunsaturated fatty acids (n-3 LCPUFA). Fatty acids were quantified using gas-chromatography coupled with flame ionization detection (GC-FID), and the distribution of different fatty acids within lipid classes (neutral and polar lipids) was achieved after oil extraction using the Folch method and separation of lipid classes via solid-phase extraction for further GC-FID analysis. Red cusk-eel was the fish species with the lowest content of both EPA and DHA (40.8 and 74.4 mg/100 g, respectively) whereas mackerel contained the highest amount (414.7 and 956.0 mg/100 g for EPA and DHA, respectively). -

Concholepas Hemocyanin Biosynthesis Takes Place in the Hepatopancreas, with Hemocytes Being Involved in Its Metabolism
Cell Tissue Res DOI 10.1007/s00441-010-1057-6 REGULAR ARTICLE Concholepas hemocyanin biosynthesis takes place in the hepatopancreas, with hemocytes being involved in its metabolism Augusto Manubens & Fabián Salazar & Denise Haussmann & Jaime Figueroa & Miguel Del Campo & Jonathan Martínez Pinto & Laura Huaquín & Alejandro Venegas & María Inés Becker Received: 5 May 2010 /Accepted: 8 September 2010 # Springer-Verlag 2010 Abstract Hemocyanins are copper-containing glycopro- them from hemolymph. We identified three types of teins in some molluscs and arthropods, and their best- granular cells. The most abundant type was a phagocyte- known function is O2 transport. We studied the site of their like cell with small cytoplasmic granules. The second type biosynthesis in the gastropod Concholepas concholepas by contained large electron-dense granules. The third type had using immunological and molecular genetic approaches. vacuoles containing hemocyanin molecules suggesting that We performed immunohistochemical staining of various synthesis or catabolism occurred inside these cells. Our organs, including the mantle, branchia, and hepatopancreas, failure to detect cch-mRNA in hemocytes by reverse and detected C. concholepas hemocyanin (CCH) molecules transcription with the polymerase chain reaction (RT-PCR) in circulating and tissue-associated hemocytes by electron led us to propose that hemocytes instead played a role in microscopy. To characterize the hemocytes, we purified CCH metabolism. This hypothesis was supported by colloidal gold staining showing hemocyanin molecules in This study was supported in part by Fundación COPEC-PUC SC0014, electron-dense granules inside hemocytes. RT-PCR analy- QC057 and FONDECYT no. 105-0150 grants. sis, complemented by in situ hybridization analyses with : : : : A. Manubens F.