Occurrence of Nematode – Antagonistic Fungi And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pdf (464.93 K)
Egypt. J. Agric. Res., 89 (2), 2011 535 POPULATION DYNAMICS OF THE GREEN SHIELD SCALE, PULVINARIA PSIDII (HEMIPTERA : COCCIDAE) ON GUAVA TREES AT SHIBIN EL-QANATER DISTRICT, QALUBIYA GOVERNORATE, EGYPT ELWAN, E. A., A. M. SERAG AND MAHA I. EL-SAYED Plant Protection Research Institute, ARC, Dokki, Giza (Manuscript received 5 January, 2011) Abstract The population dynamics of the green shield scale, Pulvinaria psidii (Mask.) (Hemiptera - Coccidae) was studied for two successive years (2008-2009) on guava trees at Shibin El-Qanater district, Qalubiya Governorate. The obtained results revealed that, P. psidii occurred on guava trees all the year round and has two overlapping generations a year. The 1st generation started from early March to early August/mid-August, peaked in mid-May (early summer) with duration of 5.0 - 5.5 months at field conditions of 20.7 - 21.3°C and 70.7 - 71.9%R.H. The 2nd generation occurred from early May to mid-November, peaked in mid-August (late summer) with duration of 6.0 - 6.5 months at 24.2 - 25.0°C and 69.4 - 70.4%R.H., respectively. The favorable time for abundance of P. psidii occurred in early and late summer during both high temperature and relative humidity. The adult population was relatively higher than nymphal population one in winter months and this may be due to the cold weather and most of the nymphs attained to the adult stage which sheltered on stems bark or in the stem cracks. Daily mean temperature and %R.H. were effective on both nymph and adult populations in 1st and 2nd generations in the two studied years, the population was correlated with the increase of temperature. -

Thèse De Doctorat
AIX-MARSEILLE UNIVERSITÉ Ecole Doctorale Sciences de la Vie et de la Santé THÈSE DE DOCTORAT Spécialité: Génétique Presenté par: Le HE Pour obtenir le grade de docteur de l’Université Aix-Marseille Interactions hôte-pathogène entre Caenorhabditis elegans et le champignon Drechmeria coniospora Soutenue le 2 décembre 2016 devant le jury composé de: Dr. Dominique Ferrandon Rapporteur Dr. Hinrich Schulenburg Rapporteur Dr. Philippe Naquet Président Dr. Eric Record Invité Dr. Jonathan Ewbank Directeur de thèse I TABLE OF CONTENTS Table of Figures ................................................................................................................ IV Table of Tables .................................................................................................................. V CHAPTER 1. Introduction............................................................................................ 1 1.1 Host-pathogen interactions ................................................................................... 1 1.1.1 C. elegans and its innate immunity ............................................................... 1 1.1.2 Nematophagous fungus ................................................................................. 5 1.1.3 Plant pathogenic fungi ................................................................................ 10 1.2 Fungal genetic modification ............................................................................... 17 1.2.1 Small RNA for Cross-species gene silencing ............................................ -

Mints – MISR NATIONAL TRANSPORT STUDY
No. TRANSPORT PLANNING AUTHORITY MINISTRY OF TRANSPORT THE ARAB REPUBLIC OF EGYPT MiNTS – MISR NATIONAL TRANSPORT STUDY THE COMPREHENSIVE STUDY ON THE MASTER PLAN FOR NATIONWIDE TRANSPORT SYSTEM IN THE ARAB REPUBLIC OF EGYPT FINAL REPORT TECHNICAL REPORT 11 TRANSPORT SURVEY FINDINGS March 2012 JAPAN INTERNATIONAL COOPERATION AGENCY ORIENTAL CONSULTANTS CO., LTD. ALMEC CORPORATION EID KATAHIRA & ENGINEERS INTERNATIONAL JR - 12 039 No. TRANSPORT PLANNING AUTHORITY MINISTRY OF TRANSPORT THE ARAB REPUBLIC OF EGYPT MiNTS – MISR NATIONAL TRANSPORT STUDY THE COMPREHENSIVE STUDY ON THE MASTER PLAN FOR NATIONWIDE TRANSPORT SYSTEM IN THE ARAB REPUBLIC OF EGYPT FINAL REPORT TECHNICAL REPORT 11 TRANSPORT SURVEY FINDINGS March 2012 JAPAN INTERNATIONAL COOPERATION AGENCY ORIENTAL CONSULTANTS CO., LTD. ALMEC CORPORATION EID KATAHIRA & ENGINEERS INTERNATIONAL JR - 12 039 USD1.00 = EGP5.96 USD1.00 = JPY77.91 (Exchange rate of January 2012) MiNTS: Misr National Transport Study Technical Report 11 TABLE OF CONTENTS Item Page CHAPTER 1: INTRODUCTION..........................................................................................................................1-1 1.1 BACKGROUND...................................................................................................................................1-1 1.2 THE MINTS FRAMEWORK ................................................................................................................1-1 1.2.1 Study Scope and Objectives .........................................................................................................1-1 -

ACLED) - Revised 2Nd Edition Compiled by ACCORD, 11 January 2018
EGYPT, YEAR 2015: Update on incidents according to the Armed Conflict Location & Event Data Project (ACLED) - Revised 2nd edition compiled by ACCORD, 11 January 2018 National borders: GADM, November 2015b; administrative divisions: GADM, November 2015a; Hala’ib triangle and Bir Tawil: UN Cartographic Section, March 2012; Occupied Palestinian Territory border status: UN Cartographic Sec- tion, January 2004; incident data: ACLED, undated; coastlines and inland waters: Smith and Wessel, 1 May 2015 Conflict incidents by category Development of conflict incidents from 2006 to 2015 category number of incidents sum of fatalities battle 314 1765 riots/protests 311 33 remote violence 309 644 violence against civilians 193 404 strategic developments 117 8 total 1244 2854 This table is based on data from the Armed Conflict Location & Event Data Project This graph is based on data from the Armed Conflict Location & Event (datasets used: ACLED, undated). Data Project (datasets used: ACLED, undated). EGYPT, YEAR 2015: UPDATE ON INCIDENTS ACCORDING TO THE ARMED CONFLICT LOCATION & EVENT DATA PROJECT (ACLED) - REVISED 2ND EDITION COMPILED BY ACCORD, 11 JANUARY 2018 LOCALIZATION OF CONFLICT INCIDENTS Note: The following list is an overview of the incident data included in the ACLED dataset. More details are available in the actual dataset (date, location data, event type, involved actors, information sources, etc.). In the following list, the names of event locations are taken from ACLED, while the administrative region names are taken from GADM data which serves as the basis for the map above. In Ad Daqahliyah, 18 incidents killing 4 people were reported. The following locations were affected: Al Mansurah, Bani Ebeid, Gamasa, Kom el Nour, Mit Salsil, Sursuq, Talkha. -

The Data on Periodical (Weekly) Market at the End of the 19Th Century in Egypt -The Cases of Qaliubiya, Sharqiya and Daqahliya Provinces
The Data on Periodical (Weekly) Market at the End of the 19th Century in Egypt -The cases of Qaliubiya, Sharqiya and Daqahliya Provinces Hiroshi Kato Some geographers and historians are concerned with periodical market, which they define as the place of economic transactions peculiar to so called "peasant society. In Egypt, which is, as well known, a typical hydraulic society, periodical market, that is weekly market (α1- siiq al-usbu i) in the Islamic world, still has the important economic functions in rural areas at the present, as well as it had in the past. The author is now collecting the data on Egyptian weekly market from the 19th century to the present, based upon source materials on one hand, and field research on the other. The aim of this paper is to present some statistical and ge0- graphical data on Egyptian weekly market at the end of the 19th century to the researchers who are interested in periodical market in agrarian society, before the intensive study, which the author is planning in the future, on the economic functions of Egyptian weekly market and their transformation in the process of the modernization of Egyptian society. The source material from which the data are collected is A. Boinet, Geographie Econ0- mique et Administrative de I'Egypte, Basse-Egypte I, Le Caire, 1902. It is the results of the population census in 1897 and the agrarian census maybe took in 1898 and 1899, being annexed to the population census in the previous year. The data are arranged village by village, and contain the statistics on cultivated area, crops, planted trees, animals, industry, traffic by rail- road, and transportation by the Nile and canals, and the descriptive informations and remarks on school, canal, railroad, market, post office and so on. -

3.Safwat A. Azer, Fatma S. Mohammad
Human Journals Research Article August 2019 Vol.:13, Issue:2 © All rights are reserved by Safwat A. Azer et al. Survey and Evaluation of the Weeds Associated with Winter Crops at Some Areas of Qalyubia Governorate, Egypt Keywords: Survey, Evaluation, Weeds, Winter Crops, Qalyubia governorate, Egypt ABSTRACT 1*Safwat A. Azer, 1Fatma S. Mohammad This research aims to survey and evaluate the weeds associated with winter crops at some areas of Qalyubia 1. Flora and Phytotaxonomy Researches Department, governorate. Two hundred and eighty six quadrates were selected to represent the distribution of weed species among Horticultural Research the studied crops and areas. At Al Gabal Al Asfar area, the Institute, Agricultural Research Center, Giza, Egypt. highest frequency ratio 55% of Cyperus rotundus was recorded while; the lowest 2.5 % was noticed for Leucaena Submission: 28 July 2019 leucocephala, Sesbania sesban and Solanum tuberosum. Similarly, the ratio 2.5 % was observed for Brassica nigra, Accepted: 2 August 2019 Desmostachya bipinnata, Medicago polymorpha and Rumex Published: 30 August 2019 dentatus at Abu Zaable area. The highest abundance ratio 38% of dominant weed species were recorded at Shibin El Qanater while; the lowest 4.76% was noticed at Qalyub area. Based on similarity values of weed species among the studied areas, Al Khankah and Al Gabal Al Asfar showed the highest value 0.700 while; the lowest 0.373 was noticed between Qaha and Al Gabal Al Asfar. Moreover, the similarity values www.ijsrm.humanjournals.com of weed species among studied crops showed that Egyptian clover and lettuce had the highest value 0.646 while; the lowest 0.167 was recorded between cauliflower and strawberry. -

Notizbuchartige Auswahlliste Zur Bestimmungsliteratur Für Unitunicate Pyrenomyceten, Saccharomycetales Und Taphrinales
Pilzgattungen Europas - Liste 9: Notizbuchartige Auswahlliste zur Bestimmungsliteratur für unitunicate Pyrenomyceten, Saccharomycetales und Taphrinales Bernhard Oertel INRES Universität Bonn Auf dem Hügel 6 D-53121 Bonn E-mail: [email protected] 24.06.2011 Zur Beachtung: Hier befinden sich auch die Ascomycota ohne Fruchtkörperbildung, selbst dann, wenn diese mit gewissen Discomyceten phylogenetisch verwandt sind. Gattungen 1) Hauptliste 2) Liste der heute nicht mehr gebräuchlichen Gattungsnamen (Anhang) 1) Hauptliste Acanthogymnomyces Udagawa & Uchiyama 2000 (ein Segregate von Spiromastix mit Verwandtschaft zu Shanorella) [Europa?]: Typus: A. terrestris Udagawa & Uchiyama Erstbeschr.: Udagawa, S.I. u. S. Uchiyama (2000), Acanthogymnomyces ..., Mycotaxon 76, 411-418 Acanthonitschkea s. Nitschkia Acanthosphaeria s. Trichosphaeria Actinodendron Orr & Kuehn 1963: Typus: A. verticillatum (A.L. Sm.) Orr & Kuehn (= Gymnoascus verticillatus A.L. Sm.) Erstbeschr.: Orr, G.F. u. H.H. Kuehn (1963), Mycopath. Mycol. Appl. 21, 212 Lit.: Apinis, A.E. (1964), Revision of British Gymnoascaceae, Mycol. Pap. 96 (56 S. u. Taf.) Mulenko, Majewski u. Ruszkiewicz-Michalska (2008), A preliminary checklist of micromycetes in Poland, 330 s. ferner in 1) Ajellomyces McDonough & A.L. Lewis 1968 (= Emmonsiella)/ Ajellomycetaceae: Lebensweise: Z.T. humanpathogen Typus: A. dermatitidis McDonough & A.L. Lewis [Anamorfe: Zymonema dermatitidis (Gilchrist & W.R. Stokes) C.W. Dodge; Synonym: Blastomyces dermatitidis Gilchrist & Stokes nom. inval.; Synanamorfe: Malbranchea-Stadium] Anamorfen-Formgattungen: Emmonsia, Histoplasma, Malbranchea u. Zymonema (= Blastomyces) Bestimm. d. Gatt.: Arx (1971), On Arachniotus and related genera ..., Persoonia 6(3), 371-380 (S. 379); Benny u. Kimbrough (1980), 20; Domsch, Gams u. Anderson (2007), 11; Fennell in Ainsworth et al. (1973), 61 Erstbeschr.: McDonough, E.S. u. A.L. -

LOT NUMBER RESERVE £ Postal History 1 Early Missionary Letter
1 LOT NUMBER RESERVE £ Postal history 1 Early Missionary letter dated 7 Dec 1830, Alexandria to Beyroot 59.00 2 French Consular Mail – Entire Alex to Marseilles with 40 centimes cancelled 5080 (diamond) via Paquebots de la Mediterrane (in red) and PD (in red). Reduced rate from 1866 44.00 Second Issue, 1867-69 (Nile Post numbers) 3 5-para (D8), Types 104 unused, with photograph 24.00 4 10-para (D91), used with watermark on face. ESC certificate 45.00 5 3 x 10-para (D9a, 9b, 9c), used in excellent condition with photograph 85.00 6 10-para (D9, Types 1-4), unused, includes Stone A Type 2 (D9A) 83.00 7 10-para (D9f, Types 1-4), unused, includes Stone B Type 2 unbroken O, with photograph 63.00 8 10-para (D9 Types 1-4), unused and used including Stone A 3rd State Type 2 with photograph 111.00 9 20-para (D10 Types 1-4), unused Stone A (2 dots) with photograph 72.00 10 20-para (D10i Types 1-4), unused Stone B (3 dots) with photograph 65.00 11 1-piastre (D11), 37 copies unused and used, all types. Worthy of research by a student of this issue 49.00 12 5-piastre (D 13 Types 1-4), unused, fine condition 390.00 13 5-piastre (D13 Types 1-4), used, fine condition 240.00 14 5-para (D8k) used, misplaced perforation 43.00 15 5-para (D8), yellow green, mint, very fine 19.00 16 10-para (D9j), unused, “white hole in wig” variety 29.00 17 1-piastre (D11v), unused, “inverted watermark” 19.00 18 1-piastre (D11m), unused, imperforate 22.00 Postage Due issue 1922 (Nile Post numbers) 19 PD30a, mint block of 12, overprint à cheval 186.00 20 PD30a, three mint singles, -
2 Assessment of Information Needs and Collection of Additional Data
2 Assessment of Information Needs and Collection of Additional Data 2.1 Introduction Greater Cairo Area and Population Cairo or rather the urban region of the Greater Cairo Metropolitan Area is the largest urban area in Egypt, Africa and the Middle East and amongst the most populous metropolises of the world. It occupies the 10th rank within mega cities across the world in the period between 2000 and 20152. Greater Cairo has been the centre of gravity for many of Egypt’s activities. It has grown mainly due to increased migration from rural areas, and high growth rates were witnessed during the second half of the 20th century vis-à-vis investments, economic activities, job opportunities and number of students. At the turn of the 21st century, Greater Cairo started to get its contemporary structure as a “main dense urban area” with varied socioeconomic levels encircled by the Ring Road and an “outer belt” of 8 new satellite cities as shown in Figure 2.1. In 2006, the population of Greater Cairo Area reached 17 million people spread across Cairo, Giza and Qaylobiya and the new cities listed in Table 2.1 below. The urbanization continues to progress, and the performance of the entire transport system is less than desirable, despite the massive efforts striven by the Egyptian government to tackle traffic congestion and environmental deterioration, by introducing a metro system and a comprehensive bus network. Table 2.1: New Cities around Greater Cairo- Type and Population 3 City Type Population in 2006 6 October Industrial 500,000 Al Sheikh Zayed Residential 48,000 15 May Industrial 180,000 Al Oboor Industrial 100,000 Badr Industrial 60,000 Al Shorooq Residential 62,000 New Cairo Residential 302,000 2 “World Urbanization Prospects, the 2001 Revision”, Department of Economic and Social Affairs, Population Division, United Nations Publications, UN, 2002. -
![Abdelrahman, Banan- [IGWS] Thesis 2017.Pdf](https://docslib.b-cdn.net/cover/3059/abdelrahman-banan-igws-thesis-2017-pdf-3353059.webp)
Abdelrahman, Banan- [IGWS] Thesis 2017.Pdf
The American University in Cairo School of Global Affairs and Public Policy Who Am I Without Exile? | Syrian Everyday Life in Cairo A Thesis Submitted to The Cynthia Nelson Institute for Gender and Women's Studies In partial fulfillment of the requirements for the degree of Master of Arts (M.A) in Gender and Women’s Studies in the Middle East / North Africa Specialization in Gendered Political Economies by Banan Abdelrahman under the supervision of Dr. Martina Rieker August/ 2017 RUNNING HEAD: SYRIAN EVERYDAY LIFE IN CAIRO منفى من انا دون Who Am I Without Exile? | Syrian Everyday Life in Cairo Banan Abdelrahman IGWS M.A Thesis | Gendered Political Economies Under the supervision of Dr. Martina Rieker Committee Members: Dr. Hanan Sabea & Dr. Gerda Heck American University of Cairo August 2017 Cover Photo ©- ii Banan- Abdelrahman RUNNING HEAD: SYRIAN EVERYDAY LIFE IN CAIRO The American University in Cairo School of Global Affairs and Public Policy Who Am I Without Exile? | Syrian Everyday Life in Cairo A Thesis Submitted to The Cynthia Nelson Institute for Gender and Women's Studies August 2017 In partial fulfillment of the requirements for the degree of Master of Arts (M.A) in Gender and Women’s Studies in the Middle East / North Africa Specialization in Gendered Political Economies by Banan Abdelrahman has been approved by: Dr. Martina Rieker Thesis Adviser ______________________________________________________ Affiliation ______________________________________________________ Dr. Hanan Sabea Thesis Reader ______________________________________________________ -
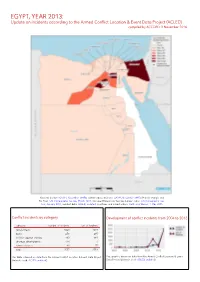
EGYPT, YEAR 2013: Update on Incidents According to the Armed Conflict Location & Event Data Project (ACLED) Compiled by ACCORD, 3 November 2016
EGYPT, YEAR 2013: Update on incidents according to the Armed Conflict Location & Event Data Project (ACLED) compiled by ACCORD, 3 November 2016 National borders: GADM, November 2015b; administrative divisions: GADM, November 2015a; Hala’ib triangle and Bir Tawil: UN Cartographic Section, March 2012; Occupied Palestinian Territory border status: UN Cartographic Sec- tion, January 2004; incident data: ACLED, undated; coastlines and inland waters: Smith and Wessel, 1 May 2015 Conflict incidents by category Development of conflict incidents from 2004 to 2013 category number of incidents sum of fatalities riots/protests 1821 1319 battle 279 495 violence against civilians 193 669 strategic developments 174 1 remote violence 90 75 total 2557 2559 This table is based on data from the Armed Conflict Location & Event Data Project This graph is based on data from the Armed Conflict Location & Event (datasets used: ACLED, undated). Data Project (datasets used: ACLED, undated). EGYPT, YEAR 2013: UPDATE ON INCIDENTS ACCORDING TO THE ARMED CONFLICT LOCATION & EVENT DATA PROJECT (ACLED) COMPILED BY ACCORD, 3 NOVEMBER 2016 LOCALIZATION OF CONFLICT INCIDENTS Note: The following list is an overview of the incident data included in the ACLED dataset. More details are available in the actual dataset (date, location data, event type, involved actors, information sources, etc.). In the following list, the names of event locations are taken from ACLED, while the administrative region names are taken from GADM data which serves as the basis for the map above. In Ad Daqahliyah, 60 incidents killing 27 people were reported. The following locations were affected: Al Mansurah, Matariya, Mit Ghamr. In Al Bahr al Ahmar, 3 incidents killing 0 people were reported. -

MINISTRY of TRANSPORT EGYPTIAN NATIONAL RAILWAYS Public Disclosure Authorized EGYPTIAN NATIONAL RAILWAYS RECONSTRUCTION PROJECT
E1997 v1 MINISTRY OF TRANSPORT EGYPTIAN NATIONAL RAILWAYS Public Disclosure Authorized EGYPTIAN NATIONAL RAILWAYS RECONSTRUCTION PROJECT ENVIRONMENTAL IMPACT AASSESSMENT Public Disclosure Authorized Public Disclosure Authorized Environmental Quality International Public Disclosure Authorized 18 El Mansour Mohamed St., Zamalek, Cairo 11211, Egypt April 2008 EGYPTIAN NATIONAL RAILWAYS EGYPTIAN NATIONAL RAILWAYS RESTRUCTURING PROJECT Environmental and Social Impact Assessment Report Environmental Quality International 18 El Mansour Mohamed St., Zamalek, Cairo 11211, Egypt May 2008 Environmental Quality International 1 Environmental Impact Assessment of ENRRP ESIA Report TABLE OF CONTENTS 1.0 Executive Summary...................................................................................................... 6 1.1 Project Objectives and Description....................................................................... 6 1.2 Legal and Institutional Aspects ............................................................................. 7 1.2.1 Relevant Egyptian Legislations ......................................................................... 7 1.2.2 Relevant World Bank Policies......................................................................... 10 1.3 Analysis of Alternatives........................................................................................ 10 1.4 Significant Impacts ............................................................................................... 13 1.4.1 Socio-economic impact...................................................................................