Asamura NEWS June, 2015
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nitrate Prodrugs Able to Release Nitric Oxide in a Controlled and Selective
Europäisches Patentamt *EP001336602A1* (19) European Patent Office Office européen des brevets (11) EP 1 336 602 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.7: C07C 205/00, A61K 31/00 20.08.2003 Bulletin 2003/34 (21) Application number: 02425075.5 (22) Date of filing: 13.02.2002 (84) Designated Contracting States: (71) Applicant: Scaramuzzino, Giovanni AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU 20052 Monza (Milano) (IT) MC NL PT SE TR Designated Extension States: (72) Inventor: Scaramuzzino, Giovanni AL LT LV MK RO SI 20052 Monza (Milano) (IT) (54) Nitrate prodrugs able to release nitric oxide in a controlled and selective way and their use for prevention and treatment of inflammatory, ischemic and proliferative diseases (57) New pharmaceutical compounds of general effects and for this reason they are useful for the prep- formula (I): F-(X)q where q is an integer from 1 to 5, pref- aration of medicines for prevention and treatment of in- erably 1; -F is chosen among drugs described in the text, flammatory, ischemic, degenerative and proliferative -X is chosen among 4 groups -M, -T, -V and -Y as de- diseases of musculoskeletal, tegumental, respiratory, scribed in the text. gastrointestinal, genito-urinary and central nervous sys- The compounds of general formula (I) are nitrate tems. prodrugs which can release nitric oxide in vivo in a con- trolled and selective way and without hypotensive side EP 1 336 602 A1 Printed by Jouve, 75001 PARIS (FR) EP 1 336 602 A1 Description [0001] The present invention relates to new nitrate prodrugs which can release nitric oxide in vivo in a controlled and selective way and without the side effects typical of nitrate vasodilators drugs. -

(2006.01) (84) Designated States (Unless Otherwise Indicated, For
) ( (51) International Patent Classification: ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, A61K 47/68 (2017.01) A61P 35/00 (2006.01) NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, SD, SE, SG, SK, SL, ST, SV, SY, TH, TJ, TM, TN, (21) International Application Number: TR, TT, TZ, UA, UG, US, UZ, VC, VN, WS, ZA, ZM, ZW. PCT/EP2020/070149 (84) Designated States (unless otherwise indicated, for every (22) International Filing Date: kind of regional protection available) . ARIPO (BW, GH, 16 July 2020 (16.07.2020) GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, (25) Filing Language: English UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, (26) Publication Language: English EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, LV, (30) Priority Data: MC, MK, MT, NL, NO, PL, PT, RO, RS, SE, SI, SK, SM, 19187692.9 23 July 2019 (23.07.2019) EP TR), OAPI (BF, BJ, CF, CG, Cl, CM, GA, GN, GQ, GW, KM, ML, MR, NE, SN, TD, TG). (71) Applicants: BAYER PHARMA AKTIENGESEL- LSCHAFT [DE/DE]; Mullerstr. 178, 13353 Berlin (DE). Declarations under Rule 4.17: BAYER AKTIENGESELLSCHAFT [DE/DE]; Kaiser- — as to applicant's entitlement to apply for and be granted a Wilhelm-Allee 1, 51373 Leverkusen (DE). patent (Rule 4.17(H)) (72) Inventors: BOHNKE, Niels; Sachsische Str. 41, 10713 Published: Berlin (DE). GRIEBENOW, Nils; Kurfurstenstr. -
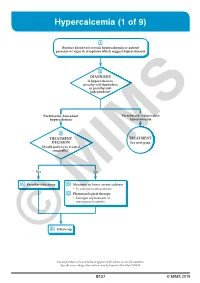
Hypercalcemia (1 of 9)
Hypercalcemia (1 of 9) 1 Routine blood test reveals hypercalcemia or patient presents w/ signs & symptoms which suggest hypercalcemia 2 DIAGNOSIS Is hypercalcemia parathyroid-dependent or parathyroid- independent? Parathyroid-dependent Parathyroid-independent hypercalcemia hypercalcemia 3 TREATMENT TREATMENT DECISION See next page Should patient be treated surgically? Yes No A Parathyroidectomy B Measures to lower serum calcium • In asymptomatic patients C Pharmacological therapy • Estrogen replacement in menopausalMIMS patients D © Follow-up Not all products are available or approved for above use in all countries. Specifi c prescribing information may be found in the latest MIMS. B127 © MIMS 2019 Hypercalcemia (2 of 9) PARATHYROID INDEPENDENT HYPERCALCEMIA 2 HYPERCALCEMIA DIAGNOSIS Determine etiology HYPERCALCEMIA VITAMIN D GRANULOMATOUS OF MALIGNANCY INTOXICATION DISEASES • Usually presents as • eg Sarcoidosis severe hypercalcemia B Measures to lower serum calcium B Measures to lower serum calcium C Pharmacological therapy C Pharmacological therapy • Loop diuretics • Corticosteroid hormones • Bisphosphonates • Calcitonin • Corticosteroid hormones 1 HYPERCALCEMIA • Normal serum Ca level: 8-10 mg/dL (2-2.5 mmol/L) • Hypercalcemia: Serum Ca >10.5 mg/dL (>2.5 mmol/L) - Use total serum Ca level corrected for albumin concentration, by adding 0.8 mg/dL to the total serum Ca level for every 1 g/dL drop in serum albumin <4 g/dL Signs & Symptoms Mild Hypercalcemia • Usually asymptomatic More Severe Hypercalcemia • Symptoms usually become more -

Adhesive Preparation
(19) & (11) EP 2 062 584 A1 (12) EUROPEAN PATENT APPLICATION published in accordance with Art. 153(4) EPC (43) Date of publication: (51) Int Cl.: 27.05.2009 Bulletin 2009/22 A61K 31/663 (2006.01) A61K 9/70 (2006.01) A61K 47/06 (2006.01) A61K 47/08 (2006.01) (2006.01) (2006.01) (21) Application number: 07807007.5 A61K 47/10 A61K 47/14 A61K 47/32 (2006.01) A61P 1/02 (2006.01) (2006.01) (2006.01) (22) Date of filing: 10.09.2007 A61P 3/14 A61P 19/00 A61P 19/02 (2006.01) A61P 19/10 (2006.01) A61P 29/00 (2006.01) A61P 35/00 (2006.01) A61P 35/02 (2006.01) A61P 35/04 (2006.01) (86) International application number: PCT/JP2007/067597 (87) International publication number: WO 2008/032678 (20.03.2008 Gazette 2008/12) (84) Designated Contracting States: • HAYASHI, Noriyuki AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Imizu-shi HU IE IS IT LI LT LU LV MC MT NL PL PT RO SE Toyama 939-0351 (JP) SI SK TR • SAKAI, Yoshiki Designated Extension States: Mishima-gun AL BA HR MK RS Osaka 618-8585 (JP) (30) Priority: 11.09.2006 JP 2006245965 (74) Representative: Keller, Günter et al Lederer & Keller (71) Applicant: Kyukyu Pharmaceutical Co., Ltd. Patentanwälte Tokyo 103-0023 (JP) Prinzregentenstrasse 16 80538 München (DE) (72) Inventors: • YAMAZAKI, Yuuhiro Imizu-shi Toyama 939-0351 (JP) (54) ADHESIVE PREPARATION (57) The present invention provides an adhesive of either of the bisphosphonic acid derivative or the salt, preparation having a plaster layer disposed on a support, a solubilizer for the active ingredient, propylene glycol, a the adhesive preparation comprising at least one active hydrogenated terpene resin, an adhesive base, and a ingredient selected from the group consisting of a bi- softening agent in the plaster layer. -

(12) Patent Application Publication (10) Pub. No.: US 2017/0119801 A1 Tabuteau (43) Pub
US 201701 19801A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0119801 A1 Tabuteau (43) Pub. Date: May 4, 2017 (54) COMPOSITIONS FOR ORAL filed on May 15, 2012, provisional application No. ADMINISTRATION OF ZOLEDRONIC ACID 61/654,292, filed on Jun. 1, 2012, provisional appli OR RELATED COMPOUNDS FOR cation No. 61/654,383, filed on Jun. 1, 2012, provi TREATING COMPLEX REGIONAL PAIN sional application No. 61/655,527, filed on Jun. 5, SYNDROME 2012, provisional application No. 61/655,541, filed on Jun. 5, 2012, provisional application No. 61/764, (71) Applicant: ANTECIP BIOVENTURES II LLC, 563, filed on Feb. 14, 2013, provisional application New York, NY (US) No. 61/762,225, filed on Feb. 7, 2013, provisional application No. 61/767,647, filed on Feb. 21, 2013, (72) Inventor: Herriot Tabuteau, New York, NY (US) provisional application No. 61/767,676, filed on Feb. 21, 2013, provisional application No. 61/803,721, (21) Appl. No.: 15/408,783 filed on Mar. 20, 2013. (22) Filed: Jan. 18, 2017 Publication Classification Related U.S. Application Data (51) Int. Cl. (63) Continuation-in-part of application No. 15/055.386, A63/675 (2006.01) filed on Feb. 26, 2016, which is a continuation of A6II 47/12 (2006.01) application No. 14/635,857, filed on Mar. 2, 2015, A6IR 9/00 (2006.01) now Pat. No. 9,283,239, which is a continuation of (52) U.S. Cl. application No. 14/279,232, filed on May 15, 2014, CPC .......... A61 K3I/675 (2013.01); A61K 9/0053 now Pat. -

Stembook 2018.Pdf
The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 WHO/EMP/RHT/TSN/2018.1 © World Health Organization 2018 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization. Suggested citation. The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances. Geneva: World Health Organization; 2018 (WHO/EMP/RHT/TSN/2018.1). Licence: CC BY-NC-SA 3.0 IGO. Cataloguing-in-Publication (CIP) data. -

A Abacavir Abacavirum Abakaviiri Abagovomab Abagovomabum
A abacavir abacavirum abakaviiri abagovomab abagovomabum abagovomabi abamectin abamectinum abamektiini abametapir abametapirum abametapiiri abanoquil abanoquilum abanokiili abaperidone abaperidonum abaperidoni abarelix abarelixum abareliksi abatacept abataceptum abatasepti abciximab abciximabum absiksimabi abecarnil abecarnilum abekarniili abediterol abediterolum abediteroli abetimus abetimusum abetimuusi abexinostat abexinostatum abeksinostaatti abicipar pegol abiciparum pegolum abisipaaripegoli abiraterone abirateronum abirateroni abitesartan abitesartanum abitesartaani ablukast ablukastum ablukasti abrilumab abrilumabum abrilumabi abrineurin abrineurinum abrineuriini abunidazol abunidazolum abunidatsoli acadesine acadesinum akadesiini acamprosate acamprosatum akamprosaatti acarbose acarbosum akarboosi acebrochol acebrocholum asebrokoli aceburic acid acidum aceburicum asebuurihappo acebutolol acebutololum asebutololi acecainide acecainidum asekainidi acecarbromal acecarbromalum asekarbromaali aceclidine aceclidinum aseklidiini aceclofenac aceclofenacum aseklofenaakki acedapsone acedapsonum asedapsoni acediasulfone sodium acediasulfonum natricum asediasulfoninatrium acefluranol acefluranolum asefluranoli acefurtiamine acefurtiaminum asefurtiamiini acefylline clofibrol acefyllinum clofibrolum asefylliiniklofibroli acefylline piperazine acefyllinum piperazinum asefylliinipiperatsiini aceglatone aceglatonum aseglatoni aceglutamide aceglutamidum aseglutamidi acemannan acemannanum asemannaani acemetacin acemetacinum asemetasiini aceneuramic -

Florencio Zaragoza Dörwald Lead Optimization for Medicinal Chemists
Florencio Zaragoza Dorwald¨ Lead Optimization for Medicinal Chemists Related Titles Smith, D. A., Allerton, C., Kalgutkar, A. S., Curry, S. H., Whelpton, R. van de Waterbeemd, H., Walker, D. K. Drug Disposition and Pharmacokinetics and Metabolism Pharmacokinetics in Drug Design From Principles to Applications 2012 2011 ISBN: 978-3-527-32954-0 ISBN: 978-0-470-68446-7 Gad, S. C. (ed.) Rankovic, Z., Morphy, R. Development of Therapeutic Lead Generation Approaches Agents Handbook in Drug Discovery 2012 2010 ISBN: 978-0-471-21385-7 ISBN: 978-0-470-25761-6 Tsaioun, K., Kates, S. A. (eds.) Han, C., Davis, C. B., Wang, B. (eds.) ADMET for Medicinal Chemists Evaluation of Drug Candidates A Practical Guide for Preclinical Development 2011 Pharmacokinetics, Metabolism, ISBN: 978-0-470-48407-4 Pharmaceutics, and Toxicology 2010 ISBN: 978-0-470-04491-9 Sotriffer, C. (ed.) Virtual Screening Principles, Challenges, and Practical Faller, B., Urban, L. (eds.) Guidelines Hit and Lead Profiling 2011 Identification and Optimization ISBN: 978-3-527-32636-5 of Drug-like Molecules 2009 ISBN: 978-3-527-32331-9 Florencio Zaragoza Dorwald¨ Lead Optimization for Medicinal Chemists Pharmacokinetic Properties of Functional Groups and Organic Compounds The Author All books published by Wiley-VCH are carefully produced. Nevertheless, authors, Dr. Florencio Zaragoza D¨orwald editors, and publisher do not warrant the Lonza AG information contained in these books, Rottenstrasse 6 including this book, to be free of errors. 3930 Visp Readers are advised to keep in mind that Switzerland statements, data, illustrations, procedural details or other items may inadvertently be Cover illustration: inaccurate. -

Harmonized Tariff Schedule of the United States (2004) -- Supplement 1 Annotated for Statistical Reporting Purposes
Harmonized Tariff Schedule of the United States (2004) -- Supplement 1 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2004) -- Supplement 1 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABACAVIR 136470-78-5 ACEXAMIC ACID 57-08-9 ABAFUNGIN 129639-79-8 ACICLOVIR 59277-89-3 ABAMECTIN 65195-55-3 ACIFRAN 72420-38-3 ABANOQUIL 90402-40-7 ACIPIMOX 51037-30-0 ABARELIX 183552-38-7 ACITAZANOLAST 114607-46-4 ABCIXIMAB 143653-53-6 ACITEMATE 101197-99-3 ABECARNIL 111841-85-1 ACITRETIN 55079-83-9 ABIRATERONE 154229-19-3 ACIVICIN 42228-92-2 ABITESARTAN 137882-98-5 ACLANTATE 39633-62-0 ABLUKAST 96566-25-5 ACLARUBICIN 57576-44-0 ABUNIDAZOLE 91017-58-2 ACLATONIUM NAPADISILATE 55077-30-0 ACADESINE 2627-69-2 ACODAZOLE 79152-85-5 ACAMPROSATE 77337-76-9 ACONIAZIDE 13410-86-1 ACAPRAZINE 55485-20-6 ACOXATRINE 748-44-7 ACARBOSE 56180-94-0 ACREOZAST 123548-56-1 ACEBROCHOL 514-50-1 ACRIDOREX 47487-22-9 ACEBURIC ACID 26976-72-7 -

6.6.2018 a Abacavir Abacavirum Abakaviiri Abagovomab
Lääkealan turvallisuus- ja kehittämiskeskus Säkerhets- och utvecklingscentret för läkemedelsområdet Finnish Medicines Agency 6.6. -
WO 2012/069150 A2 31 May 20 12 (31.05.2012) W P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2012/069150 A2 31 May 20 12 (31.05.2012) W P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 31/575 (2006.01) A61P 19/08 (2006.01) kind of national protection available): AE, AG, AL, AM, A61K 31/663 (2006.01) A61P 19/10 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, A61K 45/06 (2006.01) A61K 31/495 (2006.01) CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, (21) International Application Number: HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, KR, PCT/EP20 11/005721 KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, (22) International Filing Date: MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, 14 November 201 1 (14.1 1.201 1) OM, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, (25) Filing Language: English TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (26) Publication Language: English (84) Designated States (unless otherwise indicated, for every (30) Priority Data: kind of regional protection available): ARIPO (BW, GH, 10014829.5 22 November 2010 (22. 11.2010) EP GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, 11004058. -
Chemical Structure-Related Drug-Like Criteria of Global Approved Drugs
Molecules 2016, 21, 75; doi:10.3390/molecules21010075 S1 of S110 Supplementary Materials: Chemical Structure-Related Drug-Like Criteria of Global Approved Drugs Fei Mao 1, Wei Ni 1, Xiang Xu 1, Hui Wang 1, Jing Wang 1, Min Ji 1 and Jian Li * Table S1. Common names, indications, CAS Registry Numbers and molecular formulas of 6891 approved drugs. Common Name Indication CAS Number Oral Molecular Formula Abacavir Antiviral 136470-78-5 Y C14H18N6O Abafungin Antifungal 129639-79-8 C21H22N4OS Abamectin Component B1a Anthelminithic 65195-55-3 C48H72O14 Abamectin Component B1b Anthelminithic 65195-56-4 C47H70O14 Abanoquil Adrenergic 90402-40-7 C22H25N3O4 Abaperidone Antipsychotic 183849-43-6 C25H25FN2O5 Abecarnil Anxiolytic 111841-85-1 Y C24H24N2O4 Abiraterone Antineoplastic 154229-19-3 Y C24H31NO Abitesartan Antihypertensive 137882-98-5 C26H31N5O3 Ablukast Bronchodilator 96566-25-5 C28H34O8 Abunidazole Antifungal 91017-58-2 C15H19N3O4 Acadesine Cardiotonic 2627-69-2 Y C9H14N4O5 Acamprosate Alcohol Deterrant 77337-76-9 Y C5H11NO4S Acaprazine Nootropic 55485-20-6 Y C15H21Cl2N3O Acarbose Antidiabetic 56180-94-0 Y C25H43NO18 Acebrochol Steroid 514-50-1 C29H48Br2O2 Acebutolol Antihypertensive 37517-30-9 Y C18H28N2O4 Acecainide Antiarrhythmic 32795-44-1 Y C15H23N3O2 Acecarbromal Sedative 77-66-7 Y C9H15BrN2O3 Aceclidine Cholinergic 827-61-2 C9H15NO2 Aceclofenac Antiinflammatory 89796-99-6 Y C16H13Cl2NO4 Acedapsone Antibiotic 77-46-3 C16H16N2O4S Acediasulfone Sodium Antibiotic 80-03-5 C14H14N2O4S Acedoben Nootropic 556-08-1 C9H9NO3 Acefluranol Steroid