Adhesive Preparation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nitrate Prodrugs Able to Release Nitric Oxide in a Controlled and Selective
Europäisches Patentamt *EP001336602A1* (19) European Patent Office Office européen des brevets (11) EP 1 336 602 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.7: C07C 205/00, A61K 31/00 20.08.2003 Bulletin 2003/34 (21) Application number: 02425075.5 (22) Date of filing: 13.02.2002 (84) Designated Contracting States: (71) Applicant: Scaramuzzino, Giovanni AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU 20052 Monza (Milano) (IT) MC NL PT SE TR Designated Extension States: (72) Inventor: Scaramuzzino, Giovanni AL LT LV MK RO SI 20052 Monza (Milano) (IT) (54) Nitrate prodrugs able to release nitric oxide in a controlled and selective way and their use for prevention and treatment of inflammatory, ischemic and proliferative diseases (57) New pharmaceutical compounds of general effects and for this reason they are useful for the prep- formula (I): F-(X)q where q is an integer from 1 to 5, pref- aration of medicines for prevention and treatment of in- erably 1; -F is chosen among drugs described in the text, flammatory, ischemic, degenerative and proliferative -X is chosen among 4 groups -M, -T, -V and -Y as de- diseases of musculoskeletal, tegumental, respiratory, scribed in the text. gastrointestinal, genito-urinary and central nervous sys- The compounds of general formula (I) are nitrate tems. prodrugs which can release nitric oxide in vivo in a con- trolled and selective way and without hypotensive side EP 1 336 602 A1 Printed by Jouve, 75001 PARIS (FR) EP 1 336 602 A1 Description [0001] The present invention relates to new nitrate prodrugs which can release nitric oxide in vivo in a controlled and selective way and without the side effects typical of nitrate vasodilators drugs. -

Protocol Supplementary
Optimal Pharmacological Management and Prevention of Glucocorticoid-Induced Osteoporosis (GIOP) Protocol for a Systematic Review and Network Meta-Analysis Supplementary Materials: Sample Search Strategy Supplementary 1: MEDLINE Search Strategy Database: OVID Medline Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) 1946 to Present Line 1 exp Osteoporosis/ 2 osteoporos?s.ti,ab,kf. 3 Bone Diseases, Metabolic/ 4 osteop?eni*.ti,ab,kf. 5 Bone Diseases/ 6 exp Bone Resorption/ 7 malabsorption.ti,ab,kf. 8 Bone Density/ 9 BMD.ti,ab,kf. 10 exp Fractures, Bone/ 11 fracture*.ti,ab,kf. 12 (bone* adj2 (loss* or disease* or resorption* or densit* or content* or fragil* or mass* or demineral* or decalcif* or calcif* or strength*)).ti,ab,kf. 13 osteomalacia.ti,ab,kf. 14 or/1-13 15 exp Glucocorticoids/ 16 exp Steroids/ 17 (glucocorticoid* or steroid* or prednisone or prednisolone or hydrocortisone or cortisone or triamcinolone or dexamethasone or betamethasone or methylprednisolone).ti,ab,kf. 18 or/15-17 19 14 and 18 20 ((glucocorticoid-induced or glucosteroid-induced or corticosteroid-induced or glucocorticosteroid-induced) adj1 osteoporos?s).ti,ab,kf. 21 19 or 20 22 exp Diphosphonates/ 23 (bisphosphon* or diphosphon*).ti,ab,kf. 24 exp organophosphates/ or organophosphonates/ 25 (organophosphate* or organophosphonate*).ti,ab,kf. 26 (alendronate or alendronic acid or Fosamax or Binosto or Denfos or Fosagen or Lendrate).ti,ab,kf. 27 (Densidron or Adrovance or Alenotop or Alned or Dronat or Durost or Fixopan or Forosa or Fosval or Huesobone or Ostemax or Oseolen or Arendal or Beenos or Berlex or Fosalen or Fosmin or Fostolin or Fosavance).ti,ab,kf. -

(2006.01) (84) Designated States (Unless Otherwise Indicated, For
) ( (51) International Patent Classification: ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, A61K 47/68 (2017.01) A61P 35/00 (2006.01) NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, SD, SE, SG, SK, SL, ST, SV, SY, TH, TJ, TM, TN, (21) International Application Number: TR, TT, TZ, UA, UG, US, UZ, VC, VN, WS, ZA, ZM, ZW. PCT/EP2020/070149 (84) Designated States (unless otherwise indicated, for every (22) International Filing Date: kind of regional protection available) . ARIPO (BW, GH, 16 July 2020 (16.07.2020) GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, (25) Filing Language: English UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, (26) Publication Language: English EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, LV, (30) Priority Data: MC, MK, MT, NL, NO, PL, PT, RO, RS, SE, SI, SK, SM, 19187692.9 23 July 2019 (23.07.2019) EP TR), OAPI (BF, BJ, CF, CG, Cl, CM, GA, GN, GQ, GW, KM, ML, MR, NE, SN, TD, TG). (71) Applicants: BAYER PHARMA AKTIENGESEL- LSCHAFT [DE/DE]; Mullerstr. 178, 13353 Berlin (DE). Declarations under Rule 4.17: BAYER AKTIENGESELLSCHAFT [DE/DE]; Kaiser- — as to applicant's entitlement to apply for and be granted a Wilhelm-Allee 1, 51373 Leverkusen (DE). patent (Rule 4.17(H)) (72) Inventors: BOHNKE, Niels; Sachsische Str. 41, 10713 Published: Berlin (DE). GRIEBENOW, Nils; Kurfurstenstr. -

Msx-1 Is Suppressed in Bisphosphonate Exposed Jaw Bone
Msx-1 is suppressed in Bisphosphonate exposed jaw bone- analysis of bone turnover related cell signalling Falk Wehrhan, Peter Hyckel, Kerstin Amann, Jutta Ries, Philipp Stockmann, Karl Andreas Schlegel, Friedrich Wilhelm Neukam, Emeka Nkenke To cite this version: Falk Wehrhan, Peter Hyckel, Kerstin Amann, Jutta Ries, Philipp Stockmann, et al.. Msx-1 is sup- pressed in Bisphosphonate exposed jaw bone- analysis of bone turnover related cell signalling. Oral Diseases, Wiley, 2011, 17 (4), pp.433. 10.1111/j.1601-0825.2010.01778.x. hal-00618503 HAL Id: hal-00618503 https://hal.archives-ouvertes.fr/hal-00618503 Submitted on 2 Sep 2011 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Oral Diseases - Manuscript Copy Oral Diseases - Manuscript Copy Msx-1 is suppressed in Bisphosphonate exposed jaw bone- analysis of bone turnover related cell signalling Journal: Oral Diseases Manuscript ID: ODI-02-10-OM-1552.R1 Manuscript Type: Original Manuscript Date Submitted by the 07-Oct-2010 Author: Complete List of Authors: Wehrhan, Falk; University of Erlangen-Nürnberg, -

Efficacy and Safety of Elcatonin in Postmenopausal Women with Osteoporosis: a Systematic Review with Network Meta-Analysis of Randomized Clinical Trials
Osteoporosis International (2019) 30:1723–1732 https://doi.org/10.1007/s00198-019-04997-6 REVIEW Efficacy and safety of elcatonin in postmenopausal women with osteoporosis: a systematic review with network meta-analysis of randomized clinical trials W.-C. Chen1,2 & E.-Y. Lin3 & Y.-N. Kang1,4 Received: 20 November 2018 /Accepted: 21 April 2019 /Published online: 1 May 2019 # International Osteoporosis Foundation and National Osteoporosis Foundation 2019 Abstract Summary The present systematic review aimed to evaluate bone mineral density (BMD) change and complication rates of elcatonin on treating postmenopausal osteoporosis. The result confirmed efficacy of elcatonin and safety in combination thera- pies of elcatonin (C-E). Introduction Postmenopausal osteoporosis is an important issue in global aging trends. One treatment of osteoporosis is elcatonin, a kind of calcitonin. However, it has been challenged for long time because of safety. Many trials investigated on this topic, but they were designed differently. Those designs can be categorized in monotherapy of elcatonin (M-E) and C-E. Unfortunately, no synthesized evidence dealt this topic. Methods This study systematically identified target trials from six important databases and only included randomized controlled trial for synthesis. Two investigators assessed quality of eligible trials using the Cochrane Risk of Bias Tool, and they indepen- dently extracted data. Network meta-analysis performed Peto odds ratio (POR, used for dealing with zero cell) or weighted mean difference (WMD, for continuous data) with 95% confidence intervals (CI) and consistency H. Results Sixteen trials recruiting 2754 women with postmenopausal osteoporosis were included in our study. Elcatonin therapies and non-elcatonin medications had comparable fracture rates and bone mineral density change. -

HELD on 17.03.2012:- the NDAC (Analgesics, Anesthetics
1. RECOMMENDATIONS OF THE NDAC (ANALGESICS, ANESTHETICS AND RHEUMATOLOGY) HELD ON 17.03.2012:- The NDAC (Analgesics, Anesthetics and Rheumatology) deliberated the proposals on 17.03.2012 and recommended the following:- AGENDA NAME OF DRUG RECOMMENDATIONS NO. New Drugs The firm has requested for 3 indications viz mild to moderate pain, dysmenorrhoea and rheumatoid arthritis and osteoarthritis. Committee recommended that the firm should conduct double blind comparative clinical study of Meclofenamate sodium with diclofenac (100mg/day) in 4 sites distributed geographically in the country. 50% of the sites should be in multispeciality hospitals 1 Meclofenamate which have their own Institutional Ethics Sodium Committee. Ethics Committee approval should be from the same area where the site is located. The firm should submit protocol etc. to the office of DCGI and DCGI may issue approval to the study. Based on the data generated, committee may consider their proposal for the three indications proposed. Committee considered the protocol for conduct of local clinical trial in Indian 2 Flupirtine-D- population. However protocol etc. should be Gluconate forwarded to the members for evaluation and recommendation to DCG(I). Committee recommended for approval of the drug in the country subject to submission of CDTL test report to DCG(I). Phase IV clinical trial should be conducted on 500 patients 3 Apixaban within 2 years. Protocol for the Phase IV trial should be submitted to DCG(I) within 3 months of approval of the drug which can be considered and approved by DCG(I). In view of cardiovascular safety concern, committee recommended for giving 4 Dexmedetomidine permission to conduct the study subject to condition that the study should be conducted in subjects aged 8 years and above. -
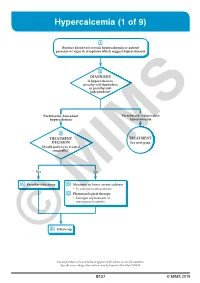
Hypercalcemia (1 of 9)
Hypercalcemia (1 of 9) 1 Routine blood test reveals hypercalcemia or patient presents w/ signs & symptoms which suggest hypercalcemia 2 DIAGNOSIS Is hypercalcemia parathyroid-dependent or parathyroid- independent? Parathyroid-dependent Parathyroid-independent hypercalcemia hypercalcemia 3 TREATMENT TREATMENT DECISION See next page Should patient be treated surgically? Yes No A Parathyroidectomy B Measures to lower serum calcium • In asymptomatic patients C Pharmacological therapy • Estrogen replacement in menopausalMIMS patients D © Follow-up Not all products are available or approved for above use in all countries. Specifi c prescribing information may be found in the latest MIMS. B127 © MIMS 2019 Hypercalcemia (2 of 9) PARATHYROID INDEPENDENT HYPERCALCEMIA 2 HYPERCALCEMIA DIAGNOSIS Determine etiology HYPERCALCEMIA VITAMIN D GRANULOMATOUS OF MALIGNANCY INTOXICATION DISEASES • Usually presents as • eg Sarcoidosis severe hypercalcemia B Measures to lower serum calcium B Measures to lower serum calcium C Pharmacological therapy C Pharmacological therapy • Loop diuretics • Corticosteroid hormones • Bisphosphonates • Calcitonin • Corticosteroid hormones 1 HYPERCALCEMIA • Normal serum Ca level: 8-10 mg/dL (2-2.5 mmol/L) • Hypercalcemia: Serum Ca >10.5 mg/dL (>2.5 mmol/L) - Use total serum Ca level corrected for albumin concentration, by adding 0.8 mg/dL to the total serum Ca level for every 1 g/dL drop in serum albumin <4 g/dL Signs & Symptoms Mild Hypercalcemia • Usually asymptomatic More Severe Hypercalcemia • Symptoms usually become more -

Clinical Effects of Switching from Minodronate to Denosumab
Kobayashi et al. BMC Women's Health (2020) 20:48 https://doi.org/10.1186/s12905-020-00913-x RESEARCH ARTICLE Open Access Clinical effects of switching from minodronate to denosumab treatment in patients with postmenopausal osteoporosis: a retrospective study Masaki Kobayashi, Kenjiro Sawada*, Akihiko Yoshimura, Misa Yamamoto, Aasa Shimizu, Kotaro Shimura, Naoko Komura, Mayuko Miyamoto, Kyoso Ishida and Tadashi Kimura Abstract Background: Denosumab is a major treatment option for patients with postmenopausal osteoporosis; however, the evidence for its use is lacking. Therefore, in this 24-month retrospective study, we aimed to evaluate the effects of switching from minodronate (MIN) to denosumab in these patients. Methods: Patients with postmenopausal osteoporosis either switched from MIN to denosumab (Group 1; n = 32) or continued MIN treatment (Group 2; n = 24). Bone mineral density (BMD) of the lumbar spine (L2–L4) and femoral neck was assessed at baseline and every 6 months for 24 months. Serum bone- specific alkaline phosphatase (BAP) and N-terminal telopeptide were measured at baseline, 12 months, and 24 months. Results: Twenty-nine of the 32 patients (90.6%) in group 1 and all patients (24/24) in group 2 completed the 24-month follow-up. Switching from MIN to denosumab (Group 1) significantly increased lumbar BMD at 12, 18, and 24 months (6.1, 7.4, and 9.6%, respectively) and femoral neck BMD at 12, 18, and 24 months (2.8, 3.2, and 3.4%, respectively), whereas MIN continuous treatment (Group 2) showed no significant difference from baseline. Switching therapy also showed a significant decrease in serum BAP from baseline to 12 and 24 months (− 19.3 and − 26.5%, respectively) and serum NTX from baseline to 12 months (− 13.1%), whereas continuous MIN treatment failed to show any significant differences from baseline. -

Osteoporosis India Drug Forecast and Market Analysis to 2022
Osteoporosis India Drug Forecast and Market Analysis to 2022 Reference Code: GDHC1054CFR Publication Date: February 2013 Executive Summary Sales for Osteoporosis in India The figure below illustrates the osteoporosis sales by drug class in India during the forecast period. The Indian osteoporosis drug market was valued at approximately $437m in 2012, and is forecast to recede Sales for Osteoporosis Drugs in India by Drug Class, 2012–2022 to $433m at a negative CAGR of 0.1% by 2022 . 2% 2012 Major drivers of market growth over this forecast period Total: $437m 20% will include: Bisphosphonates Increased investment in healthcare and facility 43% SERMs accessibility nationwide (rural and urban) PTH Strontium Calcitonin Major barriers to the growth of the osteoporosis market will include: 31% 4% Loose IP laws and guidelines 2022 3% Total: $433m 17% Price-cutting practices Bisphosphonates 42% SERMs PTH Strontium Calcitonin 35% 3% Source: GlobalData © GlobalData. This report is a licensed product and is not to be copied, reproduced, shared or resold in any form. Page 2 GDHC1054CFR / Published FEB 2013 Executive Summary What Do the Physicians Think? “The NOG guidelines that are in place in the UK and that are being implemented for harmonization throughout “[The] [b]iggest need right now is that patients just don’t Europe gives you a differing percentage based upon the want to take our drugs. And we live in this environment age of the individual, so that younger people get treated that’s just becoming increasingly poisonous as far as at lower 10-year risk and older people get treated at patient perception [is concerned]. -

Study Protocol and Statistical Analysis Plan
Phase 3 Clinical Study of KHK7580 (An Intra-Subject Dose-Adjustment Study of KHK7580 for the Treatment of Hypercalcemia in Patients with Parathyroid Carcinoma or Primary Hyperparathyroidism Who Are Unable to Undergo Parathyroidectomy or Relapse After Parathyroidectomy) Protocol Sponsor: Kyowa Hakko Kirin Co., Ltd. Protocol No.: 7580-101 Version: Created on: July 30, 2018 Protocol No.: 7580-101 Handling of this Protocol The information in this protocol is owned by the sponsor, Kyowa Hakko Kirin Co., Ltd., and is provided only to those directly involved in the study, such as the investigator and subinvestigator (including clinical study collaborators and other staff), the head of the study site, the study drug administrator, the study administration office, and the IRB. Therefore, the information in this protocol should not be disclosed or leaked to third parties who are not involved in this study. The information in this protocol cannot be copied, cited, or published without the permission of Kyowa Hakko Kirin Co., Ltd. -2- Protocol No.: 7580-101 List of Abbreviations and Definitions of Terms List of abbreviations Abbreviation Unabbreviated term eCRF Electronic case report form EDC Electronic data capture PHPT Primary hyperparathyroidism PTH Parathyroid hormone PTx Parathyroidectomy SHPT Secondary hyperparathyroidism List of definitions of terms Term Definition and explanation of term Study AMG 073 A Phase 2 Clinical Study of Cinacalcet Hydrochloride at Doses of 30 to 90 mg/day in 20000204 Patients with Parathyroid Carcinoma or Refractory -

Asamura NEWS June, 2015
Vol. 15 Asamura NEWS June, 2015 DOE may successfully work to prevent a third party from avoiding the patented process by geometric isomers. Heisei25(Wa)4040: Tokyo District Court’s Decision [Summary of Facts] maxacalcitol This reports a patent infringement lawsuit case. The plaintiff is the patentee of Japanese Patent No. 3310301, hereinafter referred to as 301 Patent. This patent relates to a process of manufacturing activity type vitamin D3 derivatives including maxacalcitol. A claim of 301 Patent relates to a process summarized by the following scheme1*. base + or B-1 B-2 B-3 wherein n is 1; each of Rl and R2 is methyl; E is an eliminating group; and R10 reducing agent and R11 are independently hydrogen, …, or protected hydroxyl. 1 To simplify, the actual claim is modified. URL: http://www.asamura.jp/en/ Email: [email protected] 1 / 7 Vol. 15 (June, 2015) The plaintiff also owned a patent right which covered maxacalcitol per se, but the term of the patent right expired in December, 2010. The plaintiff has sold a pharmaceutical product for treatment of keratosis containing maxacalcitol as an active substance. The defendants on the other hand received an approval for manufacturing and selling a pharmaceutical product containing maxacalcitol in August 2012 and has imported and sold the product. The accused product has been manufactured by the following process. base reducing agent + Comparing the patented process with the accused process, the latter is different from the former in that the starting material and intermediates have a trans STEP Ⅲ configuration as emphasized by a red circle and in involving a converting step of the trans-form into the corresponding cis-one (STEP III). -

Minodronate for the Treatment of Osteoporosis
Journal name: Therapeutics and Clinical Risk Management Article Designation: Review Year: 2018 Volume: 14 Therapeutics and Clinical Risk Management Dovepress Running head verso: Ohishi and Matsuyama Running head recto: Minodronate for osteoporosis open access to scientific and medical research DOI: 149236 Open Access Full Text Article REVIEW Minodronate for the treatment of osteoporosis Tsuyoshi Ohishi1 Abstract: Minodronate is a third-generation bisphosphonate that was developed and approved Yukihiro Matsuyama2 for clinical use in osteoporosis therapy in Japan. The mechanism of action for suppressing bone resorption is the inhibition of farnesyl pyrophosphate synthase, a key enzyme in the 1Department of Orthopaedic Surgery, Enshu Hospital, Hamamatsu, Shizuoka, mevalonic acid metabolic pathway of osteoclasts, to induce apoptosis of the cells. Minodronate Japan; 2Department of Orthopaedic is the strongest inhibitor of bone resorption among the currently available oral bisphosphonates. Surgery, Hamamatsu University School of Medicine, Hamamatsu, Shizuoka, Large randomized, placebo-controlled, double-blind clinical trials have revealed an increase in Japan bone mineral density of both the lumbar spine and femoral neck over 3 years of daily minodronate therapy and risk reduction in vertebral fractures over 2 years of therapy. The increase in bone mass and the prevention of vertebral fractures are similar to those with alendronate or risedronate. The incidence of adverse events, especially gastrointestinal disturbance, is the same as or less than that with weekly or daily alendronate or risedronate. The unique mechanism of action of minodronate via the inhibition of the P2X(2/3) receptor compared with other bisphosphonates may be an advantage in reducing low back pain in patients with osteoporosis.