Bisphosphonates in Early Breast Cancer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nitrate Prodrugs Able to Release Nitric Oxide in a Controlled and Selective
Europäisches Patentamt *EP001336602A1* (19) European Patent Office Office européen des brevets (11) EP 1 336 602 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.7: C07C 205/00, A61K 31/00 20.08.2003 Bulletin 2003/34 (21) Application number: 02425075.5 (22) Date of filing: 13.02.2002 (84) Designated Contracting States: (71) Applicant: Scaramuzzino, Giovanni AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU 20052 Monza (Milano) (IT) MC NL PT SE TR Designated Extension States: (72) Inventor: Scaramuzzino, Giovanni AL LT LV MK RO SI 20052 Monza (Milano) (IT) (54) Nitrate prodrugs able to release nitric oxide in a controlled and selective way and their use for prevention and treatment of inflammatory, ischemic and proliferative diseases (57) New pharmaceutical compounds of general effects and for this reason they are useful for the prep- formula (I): F-(X)q where q is an integer from 1 to 5, pref- aration of medicines for prevention and treatment of in- erably 1; -F is chosen among drugs described in the text, flammatory, ischemic, degenerative and proliferative -X is chosen among 4 groups -M, -T, -V and -Y as de- diseases of musculoskeletal, tegumental, respiratory, scribed in the text. gastrointestinal, genito-urinary and central nervous sys- The compounds of general formula (I) are nitrate tems. prodrugs which can release nitric oxide in vivo in a con- trolled and selective way and without hypotensive side EP 1 336 602 A1 Printed by Jouve, 75001 PARIS (FR) EP 1 336 602 A1 Description [0001] The present invention relates to new nitrate prodrugs which can release nitric oxide in vivo in a controlled and selective way and without the side effects typical of nitrate vasodilators drugs. -

(2006.01) (84) Designated States (Unless Otherwise Indicated, For
) ( (51) International Patent Classification: ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, A61K 47/68 (2017.01) A61P 35/00 (2006.01) NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, SD, SE, SG, SK, SL, ST, SV, SY, TH, TJ, TM, TN, (21) International Application Number: TR, TT, TZ, UA, UG, US, UZ, VC, VN, WS, ZA, ZM, ZW. PCT/EP2020/070149 (84) Designated States (unless otherwise indicated, for every (22) International Filing Date: kind of regional protection available) . ARIPO (BW, GH, 16 July 2020 (16.07.2020) GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, (25) Filing Language: English UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, (26) Publication Language: English EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, LV, (30) Priority Data: MC, MK, MT, NL, NO, PL, PT, RO, RS, SE, SI, SK, SM, 19187692.9 23 July 2019 (23.07.2019) EP TR), OAPI (BF, BJ, CF, CG, Cl, CM, GA, GN, GQ, GW, KM, ML, MR, NE, SN, TD, TG). (71) Applicants: BAYER PHARMA AKTIENGESEL- LSCHAFT [DE/DE]; Mullerstr. 178, 13353 Berlin (DE). Declarations under Rule 4.17: BAYER AKTIENGESELLSCHAFT [DE/DE]; Kaiser- — as to applicant's entitlement to apply for and be granted a Wilhelm-Allee 1, 51373 Leverkusen (DE). patent (Rule 4.17(H)) (72) Inventors: BOHNKE, Niels; Sachsische Str. 41, 10713 Published: Berlin (DE). GRIEBENOW, Nils; Kurfurstenstr. -

June 2011 Circular No
7 th June 2011 Circular No. P06/2011 Dear Healthcare Professional, Re: European Medicines Agency finalises review of bisphosphonates and atypical stress fractures Bisphosphonates have been authorised in the EU for hypercalcaemia and the prevention of bone problems in patients with cancer since the early 1990s. They have also been available since the mid 1990s for the treatment of osteoporosis and Paget’s disease of the bone. Bisphosphonates include alendronic acid, clodronic acid, etidronic acid, ibandronic acid, neridronic acid, pamidronic acid, risedronic acid, tiludronic acid and zoledronic acid. They are available in the EU as tablets and as solutions for infusion under various trade names and as generic medicines2. In 2008, the CHMP’s Pharmacovigilance Working Party (PhVWP) noted that alendronic acid was associated with an increased risk of atypical fracture of the femur (thigh bone) that developed with low or no trauma. As a result, a warning was added to the product information of alendronic acid-containing medicines across Europe. The PhVWP also concluded at the time that it was not possible to rule out the possibility that the effect could be a class effect (an effect common to all bisphosphonates), and decided to keep the issue under close review. In April 2010, the PhVWP noted that further data from both the published literature and post- marketing reports were now available that suggested that atypical stress fractures of the femur may be a class effect. The working party concluded that there was a need to conduct a further review to determine if any regulatory action was necessary. Page 1 of 3 Medicines Authority 203 Level 3, Rue D'Argens, Gzira, GZR 1368 – Malta. -
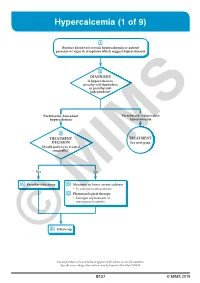
Hypercalcemia (1 of 9)
Hypercalcemia (1 of 9) 1 Routine blood test reveals hypercalcemia or patient presents w/ signs & symptoms which suggest hypercalcemia 2 DIAGNOSIS Is hypercalcemia parathyroid-dependent or parathyroid- independent? Parathyroid-dependent Parathyroid-independent hypercalcemia hypercalcemia 3 TREATMENT TREATMENT DECISION See next page Should patient be treated surgically? Yes No A Parathyroidectomy B Measures to lower serum calcium • In asymptomatic patients C Pharmacological therapy • Estrogen replacement in menopausalMIMS patients D © Follow-up Not all products are available or approved for above use in all countries. Specifi c prescribing information may be found in the latest MIMS. B127 © MIMS 2019 Hypercalcemia (2 of 9) PARATHYROID INDEPENDENT HYPERCALCEMIA 2 HYPERCALCEMIA DIAGNOSIS Determine etiology HYPERCALCEMIA VITAMIN D GRANULOMATOUS OF MALIGNANCY INTOXICATION DISEASES • Usually presents as • eg Sarcoidosis severe hypercalcemia B Measures to lower serum calcium B Measures to lower serum calcium C Pharmacological therapy C Pharmacological therapy • Loop diuretics • Corticosteroid hormones • Bisphosphonates • Calcitonin • Corticosteroid hormones 1 HYPERCALCEMIA • Normal serum Ca level: 8-10 mg/dL (2-2.5 mmol/L) • Hypercalcemia: Serum Ca >10.5 mg/dL (>2.5 mmol/L) - Use total serum Ca level corrected for albumin concentration, by adding 0.8 mg/dL to the total serum Ca level for every 1 g/dL drop in serum albumin <4 g/dL Signs & Symptoms Mild Hypercalcemia • Usually asymptomatic More Severe Hypercalcemia • Symptoms usually become more -

Adhesive Preparation
(19) & (11) EP 2 062 584 A1 (12) EUROPEAN PATENT APPLICATION published in accordance with Art. 153(4) EPC (43) Date of publication: (51) Int Cl.: 27.05.2009 Bulletin 2009/22 A61K 31/663 (2006.01) A61K 9/70 (2006.01) A61K 47/06 (2006.01) A61K 47/08 (2006.01) (2006.01) (2006.01) (21) Application number: 07807007.5 A61K 47/10 A61K 47/14 A61K 47/32 (2006.01) A61P 1/02 (2006.01) (2006.01) (2006.01) (22) Date of filing: 10.09.2007 A61P 3/14 A61P 19/00 A61P 19/02 (2006.01) A61P 19/10 (2006.01) A61P 29/00 (2006.01) A61P 35/00 (2006.01) A61P 35/02 (2006.01) A61P 35/04 (2006.01) (86) International application number: PCT/JP2007/067597 (87) International publication number: WO 2008/032678 (20.03.2008 Gazette 2008/12) (84) Designated Contracting States: • HAYASHI, Noriyuki AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Imizu-shi HU IE IS IT LI LT LU LV MC MT NL PL PT RO SE Toyama 939-0351 (JP) SI SK TR • SAKAI, Yoshiki Designated Extension States: Mishima-gun AL BA HR MK RS Osaka 618-8585 (JP) (30) Priority: 11.09.2006 JP 2006245965 (74) Representative: Keller, Günter et al Lederer & Keller (71) Applicant: Kyukyu Pharmaceutical Co., Ltd. Patentanwälte Tokyo 103-0023 (JP) Prinzregentenstrasse 16 80538 München (DE) (72) Inventors: • YAMAZAKI, Yuuhiro Imizu-shi Toyama 939-0351 (JP) (54) ADHESIVE PREPARATION (57) The present invention provides an adhesive of either of the bisphosphonic acid derivative or the salt, preparation having a plaster layer disposed on a support, a solubilizer for the active ingredient, propylene glycol, a the adhesive preparation comprising at least one active hydrogenated terpene resin, an adhesive base, and a ingredient selected from the group consisting of a bi- softening agent in the plaster layer. -

Patent Application Publication ( 10 ) Pub . No . : US 2019 / 0192440 A1
US 20190192440A1 (19 ) United States (12 ) Patent Application Publication ( 10) Pub . No. : US 2019 /0192440 A1 LI (43 ) Pub . Date : Jun . 27 , 2019 ( 54 ) ORAL DRUG DOSAGE FORM COMPRISING Publication Classification DRUG IN THE FORM OF NANOPARTICLES (51 ) Int . CI. A61K 9 / 20 (2006 .01 ) ( 71 ) Applicant: Triastek , Inc. , Nanjing ( CN ) A61K 9 /00 ( 2006 . 01) A61K 31/ 192 ( 2006 .01 ) (72 ) Inventor : Xiaoling LI , Dublin , CA (US ) A61K 9 / 24 ( 2006 .01 ) ( 52 ) U . S . CI. ( 21 ) Appl. No. : 16 /289 ,499 CPC . .. .. A61K 9 /2031 (2013 . 01 ) ; A61K 9 /0065 ( 22 ) Filed : Feb . 28 , 2019 (2013 .01 ) ; A61K 9 / 209 ( 2013 .01 ) ; A61K 9 /2027 ( 2013 .01 ) ; A61K 31/ 192 ( 2013. 01 ) ; Related U . S . Application Data A61K 9 /2072 ( 2013 .01 ) (63 ) Continuation of application No. 16 /028 ,305 , filed on Jul. 5 , 2018 , now Pat . No . 10 , 258 ,575 , which is a (57 ) ABSTRACT continuation of application No . 15 / 173 ,596 , filed on The present disclosure provides a stable solid pharmaceuti Jun . 3 , 2016 . cal dosage form for oral administration . The dosage form (60 ) Provisional application No . 62 /313 ,092 , filed on Mar. includes a substrate that forms at least one compartment and 24 , 2016 , provisional application No . 62 / 296 , 087 , a drug content loaded into the compartment. The dosage filed on Feb . 17 , 2016 , provisional application No . form is so designed that the active pharmaceutical ingredient 62 / 170, 645 , filed on Jun . 3 , 2015 . of the drug content is released in a controlled manner. Patent Application Publication Jun . 27 , 2019 Sheet 1 of 20 US 2019 /0192440 A1 FIG . -

Effects of Teriparatide and Bisphosphonate on Spinal Fusion Procedure
Effects of Teriparatide and Bisphosphonate on Spinal Fusion Procedure: A systematic review and network meta-analysis (Supplementary) Index of Supplementary Supplementary File 1. Database and search strategy Supplementary File 2. Risk of bias Supplementary File 3. Forest plot of fusion rate Supplementary File 4. Cumulative probability and SUCRA of fusion rate Supplementary File 5. Inconsistency test for network meta-analysis of fusion rate Supplementary File 6. Publication bias in network meta-analysis of fusion rate Supplementary File 7. Forest plot of ODI Supplementary File 8. Cumulative probability and SUCRA of ODI Supplementary File 9. Inconsistency test for network meta-analysis of ODI Supplementary File 10. Publication bias in network meta-analysis of ODI Supplementary File 11. Forest plot of adverse event Supplementary File 12. Cumulative probability and SUCRA of adverse event Supplementary File 13. Inconsistency test for network meta-analysis of adverse event Supplementary File 14. Publication bias in network meta-analysis of adverse event Supplementary 1 Database and search strategy Supplementary 1 Database and search strategy Database Search strategy #1. terrosu #2. forteo #3. teriparatide #4. parathyroid hormone #5. PTh #6. #1 OR #2 OR #3 OR #4 OR #5 #7. bonviva #8. alendronate #9. fosamax #10. olpadronate #11. neridronate #12. nericia #13. pamidronate #14. aredia #15. APD #16. zometa #17. avlasta #18. risedronate Primary #19. actonel search #20. boneva strategy #21. bisphosphonate #22. ibandronic #23. disambiguation #24. zoledronate #25. #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 #26. -

Tissue Engineering Approaches to the Treatment of Bisphosphonate-Related Osteonecrosis of the Jaw George Bullock
Tissue engineering approaches to the treatment of bisphosphonate-related osteonecrosis of the jaw George Bullock A thesis submitted in partial fulfilment of the requirements for the degree of Doctor of Philosophy The University of Sheffield Faculty of Engineering Department of Materials Science and Engineering March 2019 Abstract Bisphosphonate-related osteonecrosis of the jaw (BRONJ) is a disease defined by necrotic jaw bone that has become exposed through the surrounding soft tissue, which affects patients with osteoporosis and bone metastases taking the anti-resorptive bisphosphonate (BP) drugs. Currently this disease is without a specific treatment, in part due to its complex, and not fully understood, pathophysiology. This research used tissue engineering principles to further investigate the effects of BPs on the soft tissue, both in two and three dimensions, and investigated a potential preventative treatment for the disease in vitro. The BPs investigated were pamidronic acid (PA) and zoledronic acid (ZA), two BPs most commonly associated with BRONJ. We explored the effects of PA and ZA on human oral fibroblasts and keratinocytes at clinically relevant concentrations in 2D. Both PA and ZA caused significant reductions to metabolic activity, and further study indicated an increase in apoptosis in fibroblasts, and apoptosis and necrosis in keratinocytes. PA and ZA led to a significant reduction in proliferation, and ZA reduced the adhesion of keratinocytes. However, BPs did not affect cellular migration. A 3D oral mucosa model was used to investigate PA and ZA. PA prevented the stratification of newly formed epithelia and reduced the thickness of healthy epithelia. ZA showed the same effects, but at higher concentrations was also toxic. -

Asamura NEWS June, 2015
Vol. 15 Asamura NEWS June, 2015 DOE may successfully work to prevent a third party from avoiding the patented process by geometric isomers. Heisei25(Wa)4040: Tokyo District Court’s Decision [Summary of Facts] maxacalcitol This reports a patent infringement lawsuit case. The plaintiff is the patentee of Japanese Patent No. 3310301, hereinafter referred to as 301 Patent. This patent relates to a process of manufacturing activity type vitamin D3 derivatives including maxacalcitol. A claim of 301 Patent relates to a process summarized by the following scheme1*. base + or B-1 B-2 B-3 wherein n is 1; each of Rl and R2 is methyl; E is an eliminating group; and R10 reducing agent and R11 are independently hydrogen, …, or protected hydroxyl. 1 To simplify, the actual claim is modified. URL: http://www.asamura.jp/en/ Email: [email protected] 1 / 7 Vol. 15 (June, 2015) The plaintiff also owned a patent right which covered maxacalcitol per se, but the term of the patent right expired in December, 2010. The plaintiff has sold a pharmaceutical product for treatment of keratosis containing maxacalcitol as an active substance. The defendants on the other hand received an approval for manufacturing and selling a pharmaceutical product containing maxacalcitol in August 2012 and has imported and sold the product. The accused product has been manufactured by the following process. base reducing agent + Comparing the patented process with the accused process, the latter is different from the former in that the starting material and intermediates have a trans STEP Ⅲ configuration as emphasized by a red circle and in involving a converting step of the trans-form into the corresponding cis-one (STEP III). -

(12) Patent Application Publication (10) Pub. No.: US 2017/0119801 A1 Tabuteau (43) Pub
US 201701 19801A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0119801 A1 Tabuteau (43) Pub. Date: May 4, 2017 (54) COMPOSITIONS FOR ORAL filed on May 15, 2012, provisional application No. ADMINISTRATION OF ZOLEDRONIC ACID 61/654,292, filed on Jun. 1, 2012, provisional appli OR RELATED COMPOUNDS FOR cation No. 61/654,383, filed on Jun. 1, 2012, provi TREATING COMPLEX REGIONAL PAIN sional application No. 61/655,527, filed on Jun. 5, SYNDROME 2012, provisional application No. 61/655,541, filed on Jun. 5, 2012, provisional application No. 61/764, (71) Applicant: ANTECIP BIOVENTURES II LLC, 563, filed on Feb. 14, 2013, provisional application New York, NY (US) No. 61/762,225, filed on Feb. 7, 2013, provisional application No. 61/767,647, filed on Feb. 21, 2013, (72) Inventor: Herriot Tabuteau, New York, NY (US) provisional application No. 61/767,676, filed on Feb. 21, 2013, provisional application No. 61/803,721, (21) Appl. No.: 15/408,783 filed on Mar. 20, 2013. (22) Filed: Jan. 18, 2017 Publication Classification Related U.S. Application Data (51) Int. Cl. (63) Continuation-in-part of application No. 15/055.386, A63/675 (2006.01) filed on Feb. 26, 2016, which is a continuation of A6II 47/12 (2006.01) application No. 14/635,857, filed on Mar. 2, 2015, A6IR 9/00 (2006.01) now Pat. No. 9,283,239, which is a continuation of (52) U.S. Cl. application No. 14/279,232, filed on May 15, 2014, CPC .......... A61 K3I/675 (2013.01); A61K 9/0053 now Pat. -

Stembook 2018.Pdf
The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 WHO/EMP/RHT/TSN/2018.1 © World Health Organization 2018 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization. Suggested citation. The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances. Geneva: World Health Organization; 2018 (WHO/EMP/RHT/TSN/2018.1). Licence: CC BY-NC-SA 3.0 IGO. Cataloguing-in-Publication (CIP) data. -

A Abacavir Abacavirum Abakaviiri Abagovomab Abagovomabum
A abacavir abacavirum abakaviiri abagovomab abagovomabum abagovomabi abamectin abamectinum abamektiini abametapir abametapirum abametapiiri abanoquil abanoquilum abanokiili abaperidone abaperidonum abaperidoni abarelix abarelixum abareliksi abatacept abataceptum abatasepti abciximab abciximabum absiksimabi abecarnil abecarnilum abekarniili abediterol abediterolum abediteroli abetimus abetimusum abetimuusi abexinostat abexinostatum abeksinostaatti abicipar pegol abiciparum pegolum abisipaaripegoli abiraterone abirateronum abirateroni abitesartan abitesartanum abitesartaani ablukast ablukastum ablukasti abrilumab abrilumabum abrilumabi abrineurin abrineurinum abrineuriini abunidazol abunidazolum abunidatsoli acadesine acadesinum akadesiini acamprosate acamprosatum akamprosaatti acarbose acarbosum akarboosi acebrochol acebrocholum asebrokoli aceburic acid acidum aceburicum asebuurihappo acebutolol acebutololum asebutololi acecainide acecainidum asekainidi acecarbromal acecarbromalum asekarbromaali aceclidine aceclidinum aseklidiini aceclofenac aceclofenacum aseklofenaakki acedapsone acedapsonum asedapsoni acediasulfone sodium acediasulfonum natricum asediasulfoninatrium acefluranol acefluranolum asefluranoli acefurtiamine acefurtiaminum asefurtiamiini acefylline clofibrol acefyllinum clofibrolum asefylliiniklofibroli acefylline piperazine acefyllinum piperazinum asefylliinipiperatsiini aceglatone aceglatonum aseglatoni aceglutamide aceglutamidum aseglutamidi acemannan acemannanum asemannaani acemetacin acemetacinum asemetasiini aceneuramic