A Comparative Study of the Chemical Composition by SPME-GC/MS and Antiradical Activity of Less Common Citrus Species
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Citrus Group 3-1.1
CPVO-TP/203/1 Date: 18/11/2004 EUROPEAN UNION COMMUNITY PLANT VARIETY OFFICE PROTOCOL FOR DISTINCTNESS, UNIFORMITY AND STABILITY TESTS Citrus L. – Group 3 LEMONS and LIMES UPOV Species Code: CITRU, CITRU_AUR, CITRU_LAT, CITRU_LIM Adopted on 18/11/2004 1 CPVO-TP/203/1 Date: 18/11/2004 I SUBJECT OF THE PROTOCOL The protocol describes the technical procedures to be followed in order to meet the Council Regulation 2100/94 on Community Plant Variety Rights. The technical procedures have been agreed by the Administrative Council and are based on general UPOV Document TG/1/3 and UPOV guideline TG/203/1 dated 09/04/2003 for the conduct of tests for Distinctness, Uniformity and Stability. This protocol applies for all varieties of the following group of the genus Citrus L. ( Rutaceae ), and their hybrids: LEMONS AND LIMES. See below for the list of species and their subgroups: Botanical taxon Sub- Common name group Citrus assamensis S. Dutta & S.C. Bhattach. LEM Citrus aurantiifolia (Christm.) Swingle SAL Mexican Lime Citrus aurata Risso LEM Citrus balotina Poit. & Turpin LEM Citrus bergamia Risso & Poit. SAL Citrus davaoensis (Wester) Tanaka SAL Citrus duttae Tanaka LEM Citrus excelsa Wester SAL Citrus hyalopulpa Tanaka SAL Citrus jambhiri Lush. LEM Rough Lemon (RLM) Citrus javanica Blume SAL Citrus karna Raf. LEM Citrus latifolia (Yu. Tanaka) Tanaka SAL Acid Lime (LAL) Citrus limetta Risso LEM Citrus limettioides Tanaka SAL Sweet Lime (SWL) Citrus limon (L.) Burm. f. LEM Lemon Citrus limon (L.) Burm. x C. aurantifolia HLL Lemonime (Christm.) Swing. Citrus limonia Osbeck LEM Citrus longilimon Tanaka LEM Citrus longispina Wester SAL Citrus lumia Risso & Poit. -

Survey of Phenolic Compounds Produced in Citrus
USDA ??:-Z7 S rveyof Phenolic United States Department of Agriculture C mpounds Produced IliIIiI Agricultural Research In Citrus Service Technical Bulletin Number 1856 December 1998 United States Department of Agriculture Survey of Phenolic Compounds Agricultural Produced in Citrus Research Service Mark Berhow, Brent Tisserat, Katherine Kanes, and Carl Vandercook Technical Bulletin Number 1856 December 1998 This research project was conducted at USDA, Agricultural Research Service, Fruit and Vegetable Chem istry laboratory, Pasadena, California, where Berhow was a research chemist, TIsserat was a research geneticist, Kanes was a research associate, and Vandercook, now retired, was a research chemist. Berhow and Tisserat now work at the USDA-ARS National Center for AgriCUltural Utilization Research, Peoria, Illinois, where Berhow is a research chemist and Tisserat is a research geneticist. Abstract Berhow, M., B. Tisserat, K. Kanes, and C. Vandercook. 1998. Survey of Mention of trade names or companies in this publication is solely for the Phenolic Compounds Produced in Citrus. U.S. Department ofAgriculture, purpose of providing specific information and does not imply recommenda Agricultural Research Service, Technical Bulletin No. 1856, 158 pp. tion or endorsement by the U. S. Department ofAgriculture over others not mentioned. A survey of phenolic compounds, especially flavanones and flavone and flavonol compounds, using high pressure liquid chromatography was While supplies last, single copies of this publication may be obtained at no performed in Rutaceae, subfamily Aurantioideae, representing 5 genera, cost from- 35 species, and 114 cultivars. The average number of peaks, or phenolic USDA, ARS, National Center for Agricultural Utilization Research compounds, occurring in citrus leaf, flavedo, albedo, and juice vesicles 1815 North University Street were 21, 17, 15, and 9.3, respectively. -

0 Golden Hour
GOLDEN HOUR - In the hour after sunrise or the hour before sunset. In light diffused, the party in apricot hues, refused, to end. 0 CONTENTS The Japanese Garden ............................................................................................................ 2 Gin Selection ......................................................................................................................... 8 Gin Serves .......................................................................................................................... 14 Beers .................................................................................................................................... 15 Champagne and Wine Selection .............................................................................. 16 Champagne ........................................................................................................................ 17 Champagne Rosé ............................................................................................................ 19 Vodka ................................................................................................................................... 21 Teqiula ................................................................................................................................. 22 Mezcal .................................................................................................................................. 23 Pisco .................................................................................................................................... -
Holdings of the University of California Citrus Variety Collection 41
Holdings of the University of California Citrus Variety Collection Category Other identifiers CRC VI PI numbera Accession name or descriptionb numberc numberd Sourcee Datef 1. Citron and hybrid 0138-A Indian citron (ops) 539413 India 1912 0138-B Indian citron (ops) 539414 India 1912 0294 Ponderosa “lemon” (probable Citron ´ lemon hybrid) 409 539491 Fawcett’s #127, Florida collection 1914 0648 Orange-citron-hybrid 539238 Mr. Flippen, between Fullerton and Placentia CA 1915 0661 Indian sour citron (ops) (Zamburi) 31981 USDA, Chico Garden 1915 1795 Corsican citron 539415 W.T. Swingle, USDA 1924 2456 Citron or citron hybrid 539416 From CPB 1930 (Came in as Djerok which is Dutch word for “citrus” 2847 Yemen citron 105957 Bureau of Plant Introduction 3055 Bengal citron (ops) (citron hybrid?) 539417 Ed Pollock, NSW, Australia 1954 3174 Unnamed citron 230626 H. Chapot, Rabat, Morocco 1955 3190 Dabbe (ops) 539418 H. Chapot, Rabat, Morocco 1959 3241 Citrus megaloxycarpa (ops) (Bor-tenga) (hybrid) 539446 Fruit Research Station, Burnihat Assam, India 1957 3487 Kulu “lemon” (ops) 539207 A.G. Norman, Botanical Garden, Ann Arbor MI 1963 3518 Citron of Commerce (ops) 539419 John Carpenter, USDCS, Indio CA 1966 3519 Citron of Commerce (ops) 539420 John Carpenter, USDCS, Indio CA 1966 3520 Corsican citron (ops) 539421 John Carpenter, USDCS, Indio CA 1966 3521 Corsican citron (ops) 539422 John Carpenter, USDCS, Indio CA 1966 3522 Diamante citron (ops) 539423 John Carpenter, USDCS, Indio CA 1966 3523 Diamante citron (ops) 539424 John Carpenter, USDCS, Indio -

Olonisakin Adebisi Department of Chemical Sciences Adekunle Ajasin University, PMB 001
Ife Journal of Science vol. 16, no. 2 (2014) 211 COMPARATIVE STUDY OF ESSENTIAL OIL COMPOSITION OF FRESH AND DRY PEEL AND SEED OF CITRUS SINENSIS (L) OSBECK VAR SHAMUTI AND CITRUS PARADISI MACFADYEN VAR MARSH Olonisakin Adebisi Department of Chemical Sciences Adekunle Ajasin University, PMB 001. Akungba-Akoko. Ondo-State. Nigeria. E-mail: [email protected] (Received: 2nd June, 2014; Accepted:17th July, 2014 ) ABSTRACT Citrus essential oils have an impressive range of food and medicinal uses. In this study investigation has been conducted on the variation in the yield, chemical composition and their identities in oils isolated from fresh and air-dried peel and seed of orange (Citrus sinensis) and grape(Citrus paradisi) planted in a cocoa farm. The yield of solvent-extracted essential oils from the fresh peel and seed ranged between 0.31 and 1.01%, while the yield in the air-dried peel and seed of the two different citrus samples ranged between 0.98 and 2.30%. The four major compounds present in all the oils are limonene, myrcene, alpha terpinene and camphene which ranged between 74.97 - 90.58%, 5.19 - 10.41%, 0.14 - 4.00% and 0.05 - 3.87%, respectively in fresh peel and seed. In the air-dried peel and seed their values ranged between 58.64 - 77.30%, 0.08 - 5.04%, 0.05 - 3.68% and 0.02-4.88%, respectively for the four compounds. The fresh peel and seed have lower yield but contain higher percentage concentrations of major compounds that serve as compound identification for the citrus family. -

Chemical Variability of Peel and Leaf Essential Oils in the Citrus Subgenus Papeda (Swingle) and Few Relatives
plants Article Chemical Variability of Peel and Leaf Essential Oils in the Citrus Subgenus Papeda (Swingle) and Few Relatives Clémentine Baccati 1, Marc Gibernau 1, Mathieu Paoli 1 , Patrick Ollitrault 2,3 ,Félix Tomi 1,* and François Luro 2 1 Laboratoire Sciences Pour l’Environnement, Equipe Chimie et Biomasse, Université de Corse—CNRS, UMR 6134 SPE, Route des Sanguinaires, 20000 Ajaccio, France; [email protected] (C.B.); [email protected] (M.G.); [email protected] (M.P.) 2 UMR AGAP Institut, Université Montpellier, CIRAD, INRAE, Institut Agro, 20230 San Giuliano, France; [email protected] (P.O.); [email protected] (F.L.) 3 CIRAD, UMR AGAP, 20230 San Giuliano, France * Correspondence: [email protected]; Tel.: +33-495-52-4122 Abstract: The Papeda Citrus subgenus includes several species belonging to two genetically distinct groups, containing mostly little-exploited wild forms of citrus. However, little is known about the potentially large and novel aromatic diversity contained in these wild citruses. In this study, we characterized and compared the essential oils obtained from peels and leaves from representatives of both Papeda groups, and three related hybrids. Using a combination of GC, GC-MS, and 13C-NMR spectrometry, we identified a total of 60 compounds in peel oils (PO), and 76 compounds in leaf oils (LO). Limonene was the major component in almost all citrus PO, except for C. micrantha and C. hystrix, where β-pinene dominated (around 35%). LO composition was more variable, with different Citation: Baccati, C.; Gibernau, M.; major compounds among almost all samples, except for two citrus pairs: C. -
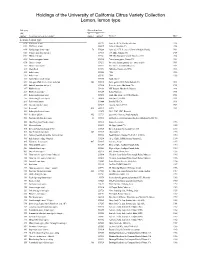
Lemon, Lemon-Type
Holdings of the University of California Citrus Variety Collection Lemon, lemon type Category Other identifiers CRC VI PI numbera Accession name or descriptionb numberc numberd Sourcee Datef Lemon, lemon type 0280 Villafranca lemon 539292 Fawcett’s #128, Florida collection 1914 0390 Villafranca lemon 600625 Grove in Glendora CA 1914 0400 Florida rough lemon (ops) 76 539268 Fawcett’s #175. Seed rec’d from A. Melson, Florida 1914 0565 Genoa lemon (Eureka type) 539313 J.W. Mills, Pomona CA 1914 0569 Millsweet lemon 539281 J.W. Mills, Pomona CA buds from tree 1195 1914 0599 Eureka variegated lemon 539325 Chase lemon grove, Corona CA 1914 0710 Chinese lemon 539211 Riverside Station grounds (see notes below) 1909 1222 Mazoe lemon (ops) 539257 A.C. Turner, Salisbury, Rhodesia 1919 2317 Limon Real 539193 Philippine Islands (via CPB) 1930 2322 India- lemon 539204 CPB 1930 2323 India lemon 539287 CPB 1930 2325 South African rough lemon 539258 South Africa? 2367 Variegated Pink Fleshed Eureka lemon 486 539315 Home garden, D.W. Field, Burbank CA 1931 2429 Amber lemon (Eureka type) 539316 Detweiler grove, Alta Loma CA 1932 2477 Khobs-el-arsa 539288 M.H. Brayard, Marrakech, Morocco 1933 2489 Rhobs-el-arsa (ops) 539289 Rabat, Morocco 1935 2544 Indian rough lemon (ops) 539290 Simla Hills, India (via CPB & Florida) 1932 2557 Gomiri rough lemon (ops) 77 103496 India (via PI, USDA) 1933 2695 Faris sweet lemon 539444 Beverly Hills CA 1938 2703 Cascade Eureka lemon 539317 Cascade Ranch 8-16-1 1939 2881 Bergamot 420 539179 UCLA 1951 2899 Italian pink fleshed lemon 133875 Fd 21, R-47, CRC, Riverside 3001 Seedless Lisbon 492 133731 Lasscock’s Nursery, South Australia 3005 Frost nucellar Eureka lemon 21 539318 2nd budded generation from sdlg of o.l. -

Lemons: Diversity and Relationships with Selected Citrus Genotypes As Measured with Nuclear Genome Markers
J. AMER. SOC. HORT. SCI. 126(3):309–317. 2001. Lemons: Diversity and Relationships with Selected Citrus Genotypes as Measured with Nuclear Genome Markers O. Gulsen1 and M.L. Roose2 Department of Botany and Plant Sciences, University of California, Riverside, CA 92521-0124 ADDITIONAL INDEX WORDS. Citrus limon, inter-simple sequence repeat, isozyme, simple sequence repeat, microsatellite markers, genetic diversity ABSTRACT. Inter-simple sequence repeats (ISSR), simple sequence repeats (SSR) and isozymes were used to measure genetic diversity and phylogenetic relationships among 95 Citrus L. accessions including 57 lemons [C. limon (L.) Burm. f.], related taxa, and three proposed ancestral species, C. maxima (Burm.) Merrill (pummelo), C. medica L. (citron), and C. reticulata Blanco (mandarin). The ancestry of lemons and several other suspected hybrids was also studied. Five isozyme and five SSR loci revealed relatively little variation among most lemons, but a high level of variation among the relatively distant Citrus taxa. Eight ISSR primers amplified a total of 103 polymorphic fragments among the 83 accessions. Similarity matrices were calculated and phylogenetic trees derived using unweighted pair-group method, arithmetic average cluster analysis. All lemons, rough lemons, and sweet lemons, as well as some other suspected hybrids, clustered with citrons. Most lemons (68%) had nearly identical marker phenotypes, suggesting they originated from a single clonal parent via a series of mutations. Citrons contributed the largest part of the lemon genome and a major part of the genomes of rough lemons, sweet lemons, and sweet limes. Bands that characterize C. reticulata and C. maxima were detected in lemons, suggesting that these taxa also contributed to the pedigree of lemon. -

Citrus Limon (Lemon) Phenomenon—A Review Of
plants Review Citrus limon (Lemon) Phenomenon—A Review of the Chemistry, Pharmacological Properties, Applications in the Modern Pharmaceutical, Food, and Cosmetics Industries, and Biotechnological Studies Marta Klimek-Szczykutowicz, Agnieszka Szopa * and Halina Ekiert Chair and Department of Pharmaceutical Botany, Jagiellonian University, Collegium Medicum, Medyczna 9, 30-688 Kraków, Poland; [email protected] (M.K.-S.); [email protected] (H.E.) * Correspondence: [email protected]; Tel.: +48-12-620-54-30 Received: 15 December 2019; Accepted: 14 January 2020; Published: 17 January 2020 Abstract: This review presents important botanical, chemical and pharmacological characteristics of Citrus limon (lemon)—a species with valuable pharmaceutical, cosmetic and culinary (healthy food) properties. A short description of the genus Citrus is followed by information on the chemical composition, metabolomic studies and biological activities of the main raw materials obtained from C. limon (fruit extract, juice, essential oil). The valuable biological activity of C. limon is determined by its high content of phenolic compounds, mainly flavonoids (e.g., diosmin, hesperidin, limocitrin) and phenolic acids (e.g., ferulic, synapic, p-hydroxybenzoic acids). The essential oil is rich in bioactive monoterpenoids such as D-limonene, β-pinene, γ-terpinene. Recently scientifically proven therapeutic activities of C. limon include anti-inflammatory, antimicrobial, anticancer and antiparasitic activities. The review pays particular attention, with references to published scientific research, to the use of C. limon in the food industry and cosmetology. It also addresses the safety of use and potential phototoxicity of the raw materials. Lastly, the review emphasizes the significance of biotechnological studies on C. -

WO 2013/077900 Al 30 May 2013 (30.05.2013) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization I International Bureau (10) International Publication Number (43) International Publication Date WO 2013/077900 Al 30 May 2013 (30.05.2013) P O P C T (51) International Patent Classification: AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, A23G 3/00 (2006.01) CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, (21) International Application Number: HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, KR, PCT/US20 12/028 148 KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, (22) International Filing Date: MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, 7 March 2012 (07.03.2012) OM, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, (25) Filing Language: English TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (26) Publication Language: English (84) Designated States (unless otherwise indicated, for every (30) Priority Data: kind of regional protection available): ARIPO (BW, GH, 13/300,990 2 1 November 201 1 (21. 11.201 1) US GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, (72) Inventor; and TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, (71) Applicant : CROWLEY, Brian [US/US]; 104 Palisades DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, Avenue, #2B, Jersey City, New Jersey 07306 (US). -

List of Citrus Fruits
Common Taxonomic SNo Notes name(s) name/constituents Yellowish-orange in colour, about the size of grapefruit and oblate in shape. 1 Amanatsu Citrus natsudaidai The fruit contains 12 segments and about 30 seeds. Balady citron 2 Palestinian Citrus medica Grown in Israel and used for Jewish ritual purposes. citron Bergamot 3 Citrus bergamia orange Bitter orange Seville orange Sour orange 4 Bigarade Citrus × aurantium orange Marmalade orange 5 Blood orange Citrus × sinensis Buddha's hand Citrus medica var. 6 Bushukan sarcodactylis Fingered citron Calamondin × Citrofortunella 7 Calamansi mitis Citrus reticulata × 8 Cam sành maxima 9 Citron Citrus medica Citrus subg. Papeda indicates the subgenus Papeda of the genus Citrus, with citrus species native to Asia.The papeda group includes some of the most Citrus subg. tropical, and also some of the most frost-tolerant citrus plants. They are 10 Papeda cultivated far less often than other citrus, though they will all hybridize with other citrus. This group contains about 15 species. 11 Clementine Citrus reticulata Corsican 12 citron Found in lowland subtropical rainforest and dry rainforest areas of Queensland and New South Wales, Australia. Early settlers consumed the 13 Desert Lime Citrus glauca fruit and retained the trees when clearing for agriculture. Commercial uses include boutique marmalade and restaurant dishes, and is exported for such. 14 Etrog Citrus medica The finger lime has been recently popularised as a gourmet bushfood. 15 Finger lime Citrus australasica Finger lime is thought to -

Citrus Sp. and Hybrids (Back to Main MBN Catalog "C")
Citrus sp. and hybrids (back to main MBN catalog "C") nice haul! Walt Steadman and the CRFG 2006 Lindcove tour we currently are not offering citrus for sale. While we feel citrus will always be part of the California home landscape, we are holding off until we see the the impact on our retail customers of pending state and federal regulations regarding Yellow Dragon Disease (Huang Long Bing, "citrus greening"). The information is provided as a free resource for professionals and home gardeners. rev 4/2015 Citrus are a large group trees and shrubs. The most commonly recognized categories (orange, lemon, grapefruit and mandarin) apparently originating in Asia from just three root species: the citron (C. medica), mandarin (C. reticulata), and pummelo (C. grandis or C. maxima). The resulting hybrids and backcrosses then radiated over thousands of years into the spectrum of hybrids and selections we now enjoy. All common citrus (exclusive of limes) appear to be hybrids and mutations of these original three types. Some, such as the mandarins, have been sold commercially for over 2300 years, while evidence of citron cultivation dates back to Babylonian times (~4000 BC). One statistic I recently heard at a UC Riverside gathering is that 60% of homes in California hav a citrus tree of some type. We offer a range of common as well as new and quite rare types. Disease Sorry folks, we have to start here. We here in California enjoy the very best quality citrus in the world because of the strict operating procedures and disease control efforts of UC Riverside, CDFA, and us commercial growers.