The Ancient Neapolitan Sweet Lime and the Calabrian Lemoncetta Locrese Belong to the Same Citrus Species
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Citrus Group 3-1.1
CPVO-TP/203/1 Date: 18/11/2004 EUROPEAN UNION COMMUNITY PLANT VARIETY OFFICE PROTOCOL FOR DISTINCTNESS, UNIFORMITY AND STABILITY TESTS Citrus L. – Group 3 LEMONS and LIMES UPOV Species Code: CITRU, CITRU_AUR, CITRU_LAT, CITRU_LIM Adopted on 18/11/2004 1 CPVO-TP/203/1 Date: 18/11/2004 I SUBJECT OF THE PROTOCOL The protocol describes the technical procedures to be followed in order to meet the Council Regulation 2100/94 on Community Plant Variety Rights. The technical procedures have been agreed by the Administrative Council and are based on general UPOV Document TG/1/3 and UPOV guideline TG/203/1 dated 09/04/2003 for the conduct of tests for Distinctness, Uniformity and Stability. This protocol applies for all varieties of the following group of the genus Citrus L. ( Rutaceae ), and their hybrids: LEMONS AND LIMES. See below for the list of species and their subgroups: Botanical taxon Sub- Common name group Citrus assamensis S. Dutta & S.C. Bhattach. LEM Citrus aurantiifolia (Christm.) Swingle SAL Mexican Lime Citrus aurata Risso LEM Citrus balotina Poit. & Turpin LEM Citrus bergamia Risso & Poit. SAL Citrus davaoensis (Wester) Tanaka SAL Citrus duttae Tanaka LEM Citrus excelsa Wester SAL Citrus hyalopulpa Tanaka SAL Citrus jambhiri Lush. LEM Rough Lemon (RLM) Citrus javanica Blume SAL Citrus karna Raf. LEM Citrus latifolia (Yu. Tanaka) Tanaka SAL Acid Lime (LAL) Citrus limetta Risso LEM Citrus limettioides Tanaka SAL Sweet Lime (SWL) Citrus limon (L.) Burm. f. LEM Lemon Citrus limon (L.) Burm. x C. aurantifolia HLL Lemonime (Christm.) Swing. Citrus limonia Osbeck LEM Citrus longilimon Tanaka LEM Citrus longispina Wester SAL Citrus lumia Risso & Poit. -

Citrus Limetta (Sweet Lemon, Mediterranean Sweet Lemon) Citrus Limetta Is a Small Tree with Lemon-Like Shape That Can Reach up to 8 M in Height
Citrus limetta (Sweet lemon, Mediterranean sweet lemon) Citrus limetta is a small tree with lemon-like shape that can reach up to 8 m in height. It has irregular branches, and relatively smooth, brownish-grey bark. It possesses numerous thorns. The leaves are more rounded and oval than an orange tree. Blossoms and new leaves are bright purple Landscape Information French Name: Limettier ﻟﻴﻤﻮﻥ ﺣﺎﻣﺾ :Arabic Name Pronounciation: SIT-rus lime-ET-ta Plant Type: Tree Origin: Asia Heat Zones: 9, 10, 11 Hardiness Zones: 10, 11, 12 Uses: Mass Planting, Container, Edible Size/Shape Tree Shape: Canopy Symmetry: Irregular Height at Maturity: 1.5 to 3 m Plant Image Spread at Maturity: 3 to 5 meters, 5 to 8 meters Citrus limetta (Sweet lemon, Mediterranean sweet lemon) Botanical Description Foliage Leaf Arrangement: Alternate Leaf Venation: Pinnate Leaf Persistance: Evergreen Leaf Type: Simple Leaf Blade: 5 - 10 cm Leaf Margins: Crenate Leaf Textures: Glossy Leaf Scent: Pleasant Color(growing season): Green Color(changing season): Green Flower Fruit Image Flower Showiness: True Flower Size Range: 1.5 - 3 Flower Scent: Pleasant Flower Color: White Seasons: Spring Trunk Trunk Esthetic Values: Smooth, Spines Fruit Fruit Type: Hesperidium Fruit Showiness: True Fruit Colors: Yellow Seasons: Spring Citrus limetta (Sweet lemon, Mediterranean sweet lemon) Horticulture Management Tolerance Frost Tolerant: No Heat Tolerant: No Drought Tolerant: Yes Salt Tolerance: Moderate Requirements Soil Requirements: Loam, Sand Soil Ph Requirements: Acidic Water Requirements: Moderate Light Requirements: Management Edible Parts: Other Image Plant Propagations: Grafting. -
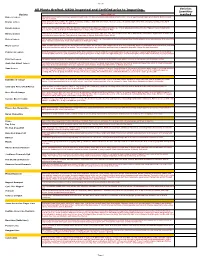
2019 Full Provisional List
Sheet1 All Plants Grafted. USDA inspected and Certified prior to Importing. Varieties Quantities Variety Description required Baboon Lemon A Brazilian lemon with very intense yellow rind and flesh. The flavour is acidic with almost a hint of lime. Tree is vigorous with large green leaves. Both tree and fruit are beautiful. Bearss Lemon 1952. Fruit closely resembles the Lisbon. Very juicy and has a high rind oil content. The leaves are a beautiful purple when first emerging, turning a nice dark green. Fruit is ready from June to December. Eureka Lemon Fruit is very juicy and highly acidic. The Eureka originated in Los Angeles, California and is one of their principal varieties. It is the "typical" lemon found in the grocery stores, nice yellow colour with typical lemon shape. Harvested November to May Harvey Lemon 1948.Having survived the disastrous deep freezes in Florida during the ’60’s and ’70’s. this varieties is known to withstand cold weather. Typical lemon shape and tart, juicy true lemon flavour. Fruit ripens in September to March. Self fertile. Zones 8A-10. Lisbon Lemon Fruit is very juicy and acic. The leaves are dense and tree is very vigorous. This Lisbon is more cold tolerant than the Eureka and is more productive. It is one of the major varieties in California. Fruit is harvested from February to May. Meyer Lemon 1908. Considered ever-bearing, the blooms are very aromatic. It is a lemon and orange hybrid. It is very cold hardy. Fruit is round with a thin rind. Fruit is juicy and has a very nice flavour, with a low acidity. -

Survey of Phenolic Compounds Produced in Citrus
USDA ??:-Z7 S rveyof Phenolic United States Department of Agriculture C mpounds Produced IliIIiI Agricultural Research In Citrus Service Technical Bulletin Number 1856 December 1998 United States Department of Agriculture Survey of Phenolic Compounds Agricultural Produced in Citrus Research Service Mark Berhow, Brent Tisserat, Katherine Kanes, and Carl Vandercook Technical Bulletin Number 1856 December 1998 This research project was conducted at USDA, Agricultural Research Service, Fruit and Vegetable Chem istry laboratory, Pasadena, California, where Berhow was a research chemist, TIsserat was a research geneticist, Kanes was a research associate, and Vandercook, now retired, was a research chemist. Berhow and Tisserat now work at the USDA-ARS National Center for AgriCUltural Utilization Research, Peoria, Illinois, where Berhow is a research chemist and Tisserat is a research geneticist. Abstract Berhow, M., B. Tisserat, K. Kanes, and C. Vandercook. 1998. Survey of Mention of trade names or companies in this publication is solely for the Phenolic Compounds Produced in Citrus. U.S. Department ofAgriculture, purpose of providing specific information and does not imply recommenda Agricultural Research Service, Technical Bulletin No. 1856, 158 pp. tion or endorsement by the U. S. Department ofAgriculture over others not mentioned. A survey of phenolic compounds, especially flavanones and flavone and flavonol compounds, using high pressure liquid chromatography was While supplies last, single copies of this publication may be obtained at no performed in Rutaceae, subfamily Aurantioideae, representing 5 genera, cost from- 35 species, and 114 cultivars. The average number of peaks, or phenolic USDA, ARS, National Center for Agricultural Utilization Research compounds, occurring in citrus leaf, flavedo, albedo, and juice vesicles 1815 North University Street were 21, 17, 15, and 9.3, respectively. -

High Biological Value Compounds Extraction from Citrus Waste with Non-Conventional Methods
foods Review High Biological Value Compounds Extraction from Citrus Waste with Non-Conventional Methods Mayra Anticona, Jesus Blesa , Ana Frigola and Maria Jose Esteve * Nutrition and Food Chemistry, University of Valencia, Avda., Vicent Andrés Estellés, s/n., 46100 Burjassot, Spain; [email protected] (M.A.); [email protected] (J.B.); [email protected] (A.F.) * Correspondence: [email protected]; Tel.: +34-963544913 Received: 27 April 2020; Accepted: 15 June 2020; Published: 20 June 2020 Abstract: Citrus fruits are extensively grown and much consumed around the world. Eighteen percent of total citrus cultivars are destined for industrial processes, and as a consequence, large amounts of waste are generated. Citrus waste is a potential source of high biological value compounds, which can be used in the food, pharmaceutical, and cosmetic industries but whose final disposal may pose a problem due to economic and environmental factors. At the same time, the emerging need to reduce the environmental impact of citrus waste and its responsible management has increased. For these reasons, the study of the use of non-conventional methods to extract high biological value compounds such as carotenoids, polyphenols, essential oils, and pectins from this type of waste has become more urgent in recent years. In this review, the effectiveness of technologies such as ultrasound assisted extraction, microwave assisted extraction, supercritical fluid extraction, pressurized water extraction, pulsed electric field, high-voltage electric discharges, and high hydrostatic pressures is described and assessed. A wide range of information concerning the principal non-conventional methods employed to obtain high-biological-value compounds from citrus waste as well as the most influencing factors about each technology are considered. -

Extraction of Pectin from Citrus Fruit Peel and Its Utilization in Preparation of Jelly
International Journal of Engineering Research & Technology (IJERT) ISSN: 2278-0181 Vol. 3 Issue 5, May - 2014 Extraction of Pectin from Citrus Fruit Peel and Its Utilization in Preparation of Jelly 1 W. Elizabeth Devi R N Shukla2 K L Bala3 A Kumar4 A A Mishra5 M.Tech in Food Technology (Food Process Engineering), K C Yadav6 Department of Food Process Engineering 2,3,4,5,6 Vaugh School of Agriculture Engineering, SHIATS Deemed Assistant Professor University Department of Food Process Engineering P.O-Naini, Allahabad, U.P-211007 Vaugh School of Agriculture Engineering, SHIATS Deemed University P.O-Naini, Allahabad, U.P-211007, India Abstract— The present study was focused on the potential of The term pectin was first described and isolated by Henry citrus peel as a source of pectin. Pectin was extracted from Sweet Braconnot in 1825 (Braconnot, 1825). Pectin is a lemon (mosambi) peel powder using two different acids (citric and polysaccharide, naturally occurring substance present in all plant nitric) and at three different temperatures, time and pH viz (60, 70 tissue. Pectin exists in varying amounts in fruit cell walls and & 80°C), (30,45 & 60 min),(1.5,2 & 2.5pH) respectively. Pectin has important nutritional and technological properties (Knox yields varied from 21.4% to 76.0% and 17.4% to 46.4% extracted by using citric acid and nitric acid respectively. The best extraction 2002). In the cell walls they serve as one of the main agents condition was found to be higher in yield by using citric acid at cementing the cellulose fibrils and may be linked covalently to 80°C, 60min, 1.5pH. -

Brazos Citrus Nursery in 2005
Variety Descriptions CITRUS Calamondin - Cold hardy into the teens. Small and sour orange fruit. Makes great marmalade. Clementine, Nules- Easy to peel with few seeds. Sweet and juicy Grapefruit, Bloomsweet - White fleshed fruit with yellow skin, easy to peel, very cold hardy Grapefruit, Cocktail - Very sweet and juicy without bitterness. Small to medium sized citrus, sweeter and less acidic than regular grapefruit. This hybrid has a dark, yellow, thin rind with a deep, yellow flesh. Great for Juicing. Grapefruit, Oro Blanco - thick, easy-to-peel rind, white flesh, juicy; the flesh is sweet to sweet tart in flavor. Grapefruit, Rio Red - fruit is large with a smooth thin skin, Yellow skin with red flesh, very juicy Grapefruit, Ruby Red- Light sweet flavor. Pink flesh. Heavy bearer that needs full sun. Kumquat, Changshou - large fruit with thick sweet skin (David’s Favorite) Kumquat, Nagami- (Sour) - bright orange, egg shaped fruit, tart flesh, very cold hardy Kumquat, Meiwa- (Sweet) - round fruit, sweet flavor, very cold hardy Lemon, Eureka Frost –vigorous spreading tree, bears multiple crops a year Lemon, Improved Meyer - compact tree, medium size fruit, thin yellow skin, very juicy Lemon, Iranian - large fruit like ponderosa, more cold hardy Lemon, Lisbon Seedless- vigorous grower, seedless & juicy Lemon, New Zealand Lemonade- sweet lemon hybrid with few seeds, very productive Lemon, Ponderosa - Large, thorny tree which produces very large fruit. Lemon, Ujukitsu - from Japan, lemon-orange cross, Ginny Shackelford juices it and serves over ice as lemonade & our good friend Gil ads something to it and calls it Ujodka! Its delicious! Lemon, Variegated Pink - beautiful green, yellow and white leaves. -
Holdings of the University of California Citrus Variety Collection 41
Holdings of the University of California Citrus Variety Collection Category Other identifiers CRC VI PI numbera Accession name or descriptionb numberc numberd Sourcee Datef 1. Citron and hybrid 0138-A Indian citron (ops) 539413 India 1912 0138-B Indian citron (ops) 539414 India 1912 0294 Ponderosa “lemon” (probable Citron ´ lemon hybrid) 409 539491 Fawcett’s #127, Florida collection 1914 0648 Orange-citron-hybrid 539238 Mr. Flippen, between Fullerton and Placentia CA 1915 0661 Indian sour citron (ops) (Zamburi) 31981 USDA, Chico Garden 1915 1795 Corsican citron 539415 W.T. Swingle, USDA 1924 2456 Citron or citron hybrid 539416 From CPB 1930 (Came in as Djerok which is Dutch word for “citrus” 2847 Yemen citron 105957 Bureau of Plant Introduction 3055 Bengal citron (ops) (citron hybrid?) 539417 Ed Pollock, NSW, Australia 1954 3174 Unnamed citron 230626 H. Chapot, Rabat, Morocco 1955 3190 Dabbe (ops) 539418 H. Chapot, Rabat, Morocco 1959 3241 Citrus megaloxycarpa (ops) (Bor-tenga) (hybrid) 539446 Fruit Research Station, Burnihat Assam, India 1957 3487 Kulu “lemon” (ops) 539207 A.G. Norman, Botanical Garden, Ann Arbor MI 1963 3518 Citron of Commerce (ops) 539419 John Carpenter, USDCS, Indio CA 1966 3519 Citron of Commerce (ops) 539420 John Carpenter, USDCS, Indio CA 1966 3520 Corsican citron (ops) 539421 John Carpenter, USDCS, Indio CA 1966 3521 Corsican citron (ops) 539422 John Carpenter, USDCS, Indio CA 1966 3522 Diamante citron (ops) 539423 John Carpenter, USDCS, Indio CA 1966 3523 Diamante citron (ops) 539424 John Carpenter, USDCS, Indio -

Improvement of Subtropical Fruit Crops: Citrus
IMPROVEMENT OF SUBTROPICAL FRUIT CROPS: CITRUS HAMILTON P. ÏRAUB, Senior Iloriiciilturist T. RALPH ROBCNSON, Senior Physiolo- gist Division of Frnil and Vegetable Crops and Diseases, Bureau of Plant Tndusiry MORE than half of the 13 fruit crops known to have been cultivated longer than 4,000 years,according to the researches of DeCandolle (7)\ are tropical and subtropical fruits—mango, oliv^e, fig, date, banana, jujube, and pomegranate. The citrus fruits as a group, the lychee, and the persimmon have been cultivated for thousands of years in the Orient; the avocado and papaya were important food crops in the American Tropics and subtropics long before the discovery of the New World. Other types, such as the pineapple, granadilla, cherimoya, jaboticaba, etc., are of more recent introduction, and some of these have not received the attention of the plant breeder to any appreciable extent. Through the centuries preceding recorded history and up to recent times, progress in the improvement of most subtropical fruits was accomplished by the trial-error method, which is crude and usually expensive if measured by modern standards. With the general accept- ance of the Mendelian principles of heredity—unit characters, domi- nance, and segregation—early in the twentieth century a starting point was provided for the development of a truly modern science of genetics. In this article it is the purpose to consider how subtropical citrus fruit crops have been improved, are now being improved, or are likel3^ to be improved by scientific breeding. Each of the more important crops will be considered more or less in detail. -

Chemical Variability of Peel and Leaf Essential Oils in the Citrus Subgenus Papeda (Swingle) and Few Relatives
plants Article Chemical Variability of Peel and Leaf Essential Oils in the Citrus Subgenus Papeda (Swingle) and Few Relatives Clémentine Baccati 1, Marc Gibernau 1, Mathieu Paoli 1 , Patrick Ollitrault 2,3 ,Félix Tomi 1,* and François Luro 2 1 Laboratoire Sciences Pour l’Environnement, Equipe Chimie et Biomasse, Université de Corse—CNRS, UMR 6134 SPE, Route des Sanguinaires, 20000 Ajaccio, France; [email protected] (C.B.); [email protected] (M.G.); [email protected] (M.P.) 2 UMR AGAP Institut, Université Montpellier, CIRAD, INRAE, Institut Agro, 20230 San Giuliano, France; [email protected] (P.O.); [email protected] (F.L.) 3 CIRAD, UMR AGAP, 20230 San Giuliano, France * Correspondence: [email protected]; Tel.: +33-495-52-4122 Abstract: The Papeda Citrus subgenus includes several species belonging to two genetically distinct groups, containing mostly little-exploited wild forms of citrus. However, little is known about the potentially large and novel aromatic diversity contained in these wild citruses. In this study, we characterized and compared the essential oils obtained from peels and leaves from representatives of both Papeda groups, and three related hybrids. Using a combination of GC, GC-MS, and 13C-NMR spectrometry, we identified a total of 60 compounds in peel oils (PO), and 76 compounds in leaf oils (LO). Limonene was the major component in almost all citrus PO, except for C. micrantha and C. hystrix, where β-pinene dominated (around 35%). LO composition was more variable, with different Citation: Baccati, C.; Gibernau, M.; major compounds among almost all samples, except for two citrus pairs: C. -

New and Noteworthy Citrus Varieties Presentation
New and Noteworthy Citrus Varieties Citrus species & Citrus Relatives Hundreds of varieties available. CITRON Citrus medica • The citron is believed to be one of the original kinds of citrus. • Trees are small and shrubby with an open growth habit. The new growth and flowers are flushed with purple and the trees are sensitive to frost. • Ethrog or Etrog citron is a variety of citron commonly used in the Jewish Feast of Tabernacles. The flesh is pale yellow and acidic, but not very juicy. The fruits hold well on the tree. The aromatic fruit is considerably larger than a lemon. • The yellow rind is glossy, thick and bumpy. Citron rind is traditionally candied for use in holiday fruitcake. Ethrog or Etrog citron CITRON Citrus medica • Buddha’s Hand or Fingered citron is a unique citrus grown mainly as a curiosity. The six to twelve inch fruits are apically split into a varying number of segments that are reminiscent of a human hand. • The rind is yellow and highly fragrant at maturity. The interior of the fruit is solid rind with no flesh or seeds. • Fingered citron fruits usually mature in late fall to early winter and hold moderately well on the tree, but not as well as other citron varieties. Buddha’s Hand or Fingered citron NAVEL ORANGES Citrus sinensis • ‘Washington navel orange’ is also known • ‘Lane Late Navel’ was the first of a as the Bahia. It was imported into the number of late maturing Australian United States in 1870. navel orange bud sport selections of Washington navel imported into • These exceptionally delicious, seedless, California. -

Application of Exogenous Spermidine Treatment for Reducing of Chilling on Fruit Quality and Quantity of Valencia Orange Var
International Journal of Farming and Allied Sciences Available online at www.ijfas.com ©2013 IJFAS Journal-2013-2-S/1292-1297 ISSN 2322-4134 ©2013 IJFAS Application of exogenous spermidine treatment for reducing of chilling on fruit quality and quantity of Valencia orange var. olinda M. Raeisi1*, R. Babadaei Samani2, M. Honarvar2 . Young Researchers and Elite club, Jiroft Branch, Islamic Azad University, Jiroft, Iran 2. Department of Horticultural Sciences, Estahban Branch, Islamic Azad University, Estahban, Iran Corresponding author: M. Raeisi ABSTRACT: With due attention to importance of citrus as one of the most important horticulture and exports crops of iran and in order to increasing of shelf life and quality improvement of citrus, research in this field is necessary, and the purpose in this study is improvement the Qualitative andquantitativecharacteristics of orange at storage via Polyamins application. This experiment was performed in factorial base on a completely randomized blocks design (CRBD) with three replications during 2010. Treatment included three levels of Spermidine (0, 1 and 1.5 mM) with control that use pure water and temperatures at two levels (2 and 4 °C) and incubation storage period at three levels (one, three and five weeks after harvest). The results indicated that the effect of Spermidine on chilling-injury, weight of single fruit, TA, flavor index (TSS/TA), fruit weight loss and TSS was significant. Effect of temperature on the mentioned characters (exceptional TA) was not significant. The effect of storage period on the percentage of fruit weight loss, vitamin C, TSS, TA, percentage of fruit juice and TSS/TA was significant.