Scientific Measurements and Errors: Determination Ofdensityof Glass
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Standard Scales SERIES 182 — Made of Low-Expansion Glass
Standard Scales SERIES 182 — Made of Low-Expansion Glass FEATURES • High-precision glass scales manufactured under Mitutoyo’s leading-edge Linear Scale 182-502-50 production technology. Technical Data • High accuracy is guaranteed to be used as Accuracy (at 20°C): (0.5+L/1000)µm, a standard for calibrating graduated scales. L = Measured length (mm) Glass material: Low expansion glass Thermal expansion coefficient: 8x10-8/K Graduation: 1mm 182-501-50 Graduation thickness: 4µm Mass: 0.75kg (250mm), 1.8kg (500mm) DIMENSIONS SPECIFICATIONS Unit: mm Metric À>`Õ>Ì ,>}i / £ Range Order No. L W T 250mm 182-501-50 280mm 20mm 10mm { 7 250mm 182-501-60* 280mm 20mm 10mm Ó À>`Õ>ÌÊÌ ViÃÃ\Ê{ 500mm 182-502-50 530mm 30mm 20mm x }iÌÊ>ÀÊÌ ViÃÃ\ÊÓä 500mm 182-502-60* 530mm 30mm 20mm *with English JCSS certificate. Working Standard Scales SERIES 182 FEATURES 182-525-10 • High-precision glass scales 182-523-10 manufactured under Mitutoyo’s leading-edge linear scale 182-522-10 Technical Data production technology. Accuracy (at 20°C): (1.5+2L/1000)µm, • Ideal for checking magnification 182-513-10 L = Measured length (mm) accuracy of profile projectors Glass material: Sodium glass Thermal expansion coefficient: 8.5x10-6/K and microscopes, and the table Graduation: 0.1mm (thickness: 20µm) feeding accuracy of measuring 0.5mm (thickness: 50µm) equipment. 1mm (thickness: 100µm) DIMENSIONS £ä Unit: mm À>`Õ>Ì £ ä°£Ê}À>`Õ>Ì ,i}i Ó°Ç ä°£Ê}À>`Õ>Ì SPECIFICATIONS Ó°x Metric ΰx ÓÓ x Range Order No. -

Drafting Machines and Parts Threof from Japan
DRAFTING MACHINES AND PARTS THEREOF FROM JAPAN Determination of the Commission in Investigation No. 731-T A-432 (Final} Under the Tariff Act of 1930, Together With the Information Obtained in the Investigation USITC PUBLICATION 2247 DECEMBER 1989 United States International Trade Commission Washington, DC 20436 UNITED STATES INTERNATIONAL TRADE COMMISSION COMMISSIONERS Anne E. Brunsdale, Chairman Ronald A. Cass, Vice Chairman Alfred E. Eckes Seeley G. Lodwick David B. Rohr Don E. Newquist Staff assigned: Elizabeth Haines, Investigator Catherine DeFilippo, Economist Marshall Wade, Financial Analyst Ruben Moller, Industry Analyst William Kane, Attorney George Deyman, Supervisory Investigator Address all communications to Kenneth R. Mason, Secretary to the Commission United States International Trade Commission Washington, DC 20436 CONTENTS Determination and Views of the Commission: Determination ..........•........... ~. .... 1 Views of the Conunission •••••••••••••.•••• ............. 3 Views of Chairman Anne E. Brunsdale •••••• . • . .. .. ... .. ... 21 Additional Views of Vice Chairman Ronald A. Cass •••• ....... • _35 Additional Views of Conunissioner Eckes ••••• .. • ......... ............ 67 Information obtained in the investigation: Introduction •••••• .................. ·• ........ A-1 Background ••••••••• ..... •· .. A-2 Nature and extent of sales at LTFV •••• .............. ............ A"."'2 The product: Description and uses .••••••••••• . .. ............. A-3 Track drafting machine •••••••. .. .. ..... ...... A-3 Band-and-pulley -

Schut for Precision
Schut for Precision Protractors / Clinometers / Spirit levels Accuracy of clinometers/spirit levels according DIN 877 Graduation Flatness (µm) µm/m " (L = length in mm) ≤ 50 ≤ 10 4 + L / 250 > 50 - 200 > 10 - 40 8 + L / 125 L > 200 > 40 16 + / 60 C08.001.EN-dealer.20110825 © 2011, Schut Geometrische Meettechniek bv 181 Measuring instruments and systems 2011/2012-D Schut.com Schut for Precision PROTRACTORS Universal digital bevel protractor This digital bevel protractor displays both decimal degrees and degrees-minutes-seconds at the same time. Measuring range: ± 360 mm. Reversible measuring direction. Resolution: 0.008° and 30". Fine adjustment. Accuracy: ± 0.08° or ± 5'. Delivery in a case with three blades (150, 200 Mode: 0 - 90°, 0 - 180° or 0 - 360°. and 300 mm), a square and an acute angle On/off switch. attachment. Reset/preset. Power supply: 1 battery type CR2032. Item No. Description Price 907.885 Bevel protractor Option: 495.157 Spare battery Single blades Item No. Blade length/mm Price 909.380 150 909.381 200 909.382 300 909.383 500 909.384 600 909.385 800 C08.302.EN-dealer.20110825 © 2011, Schut Geometrische Meettechniek bv 182 Measuring instruments and systems 2011/2012-D Schut.com Schut for Precision PROTRACTORS Universal digital bevel protractor This stainless steel, digital bevel protractor is Item No. Description Price available with blades from 150 to 1000 mm. The blades and all the measuring faces are hardened. 855.820 Bevel protractor Measuring range: ± 360°. Options: Resolution: 1', or decimal 0.01°. 495.157 Spare battery Accuracy: ± 2'. 905.409 Data cable 2 m Repeatability: 1'. -

Carpentry T-Chart
Carpentry (46.0201) T-Chart Apply geometric concepts to model and solve real world Cut trim for different shapes using degree and angles = problems Program Task: Assemble different shapes by using a PA Core Standard: CC.2.3.HS.A.14 compound miter saw; measure and compare the angles in degrees. Description: Apply geometric concepts to model and solve real world problems. Program Associated Vocabulary: Math Associated Vocabulary ANGLE, BEVEL, DEGREE, MITER, PERPENDICULAR ANGLE, DEGREES, INTERIOR ANGLES, EXTERIOR ANGLES, VERTICAL ANGLES, CORRESPONDING ANGLES, PARALLEL, TRANSVERSAL Program Formulas and Procedures: Formulas and Procedures: Carpenters often use a compound miter saw to install trim. Read angle measurement The most common angle is the 45 ° angle. Trim is installed here. Make sure you read the around doors and windows. The vertical and horizontal Reading a protractor: number that started from zero casing makes a square 90 °. The vertical and horizontal casing that is installed must be cut on a 45 ° angle. where the angle begins. (n - 2)×180 Each Corner of a Polygon = n Where n is the number of sides. Start here, where the angle begins. A square has 4 sides. (4 2) 180 Each corner = 90 4 Each angle cut = 45 ° Line up angle vertex here. Two parallel lines cut by a transversal: 1 2 m A hexagon has six sides. 3 4 (6 2) 180 Each corner = 120 6 5 n Each angle cut = 60 ° 6 7 8 l Angles 1&4, 2&3, 5&8, 6&7 are vertical angles. Angles 1&5, 2&6, 3&7, 4&8 are corresponding angles. -

PRECISION ENGINEERING TOOLS WE HAVE WHAT IT TAKES to EXCEED & EXCEL the Plant
PRECISION ENGINEERING TOOLS WE HAVE WHAT IT TAKES TO EXCEED & EXCEL The plant. The people. The passion 500,000 sq ft manufacturing | integrated research & development | advanced cnc machining | quality assurance Groz has always exceeded the expectations of tool manufacturers and users the world over. Groz carefully makes each tool under stringent quality control processes that are achieved in a hi-tech manufacturing environment in a 500,000 square foot plant. If you demand quality, trust Groz. ADDITIONS 07 08 Straight Straight & Edge Knife Edges Squares Dear Valued Customer, It is my pleasure to present to you the new catalogue that covers our 13 17 range of Precision Engineering Multi-Use Magnetic Tools. Rule and Compass Gauge We have covered fair ground over the last few years and with our state-of-the art production facility, we can now do much more 22 31 than before. You will see many Electronic Adjustable technologically superior products Edge Finders Vee Block Set as well as modifications to some of the earlier designs, in the following pages. Further, I assure you of the same top performance to which you are accustomed to from Groz. 31 35 Ball Bearing Pot We appreciate your business and Vee Block & Magnets value your loyalty & trust. Clamp Sets Warm Regards, 37 38 Sine Bars Sine Plates ANIL BAMMI Managing Director 46 49 Tweezersezers Tap Wrenchesnches - Prefessionalnal 68 7777 Rotaryry RRapidap Headd AActionct Millingng DDrillri Pressressess VicesVices Machinehine VicesVi CA02 PRECISION ENGINEERING TOOLS 1 Measuring and Marking -

Simple Picture Framing
You can make it with Simple Pictu re Framing Professional picture framing is expensive. The Triton Workcentre enables you to cut the perfect mitres needed for picture framing, and use of the Router and Jigsaw Table makes it possible to shape your own simple mouldings. This project sheet differs from the others in that no dimensions are provided. Instead, we have provided you with a range of procedural options to guide you in making the picture frame that best meets your needs. 3tD &"u Tool 1. ESSENTIAL 1. Making your picture f rame f rom purchased moulding: Triton Workcentre and your power saw, f itted with a 40 or 60 tooth blade (essential for clean mitre cuts), mitre square or combination square, hammer, nail punch, sandpaper. 2. Shaping your own mouldings: Triton Accessory Router and Jigsaw Table, your router, and a selection of decorative router cutters. 3. A jig for working on end grain is needed if you intend to strengthen the mitres by use of a spline (See the Jig Guide for details of the jig). A small handsaw will also be necessary to trim the splines. 2. USEFUL: Mitre corner clamps to aid assembly, an extension fence mounted onto Face A of the double-sided protractor, and a mitred stop block, to ensure accurate cutting to length. @ Copyright Triton Manufacturing and Design Co. Pty. Ltd. /ssue No. 2, July 1989 Construction Details Material Shoppinq List 1. WOOD Any seasoned, straight-grained timber is suitable. To determine the total length of your frame material, the standard equation is: Twice the length plus twice the width of the picture, plus eight times the width of the moulding. -

Comments on Double-Theodolite Evaluations
322 BULLETIN AMERICAN METEOROLOGICAL SOCIETY Comments on Double-Theodolite Evaluations ROBERT O. WEEDFALL AND WALTER M. JAGODZINSKI U. S. Weather Bureau (Original manuscript received 7 May 1960; revised manuscript received 26 August 1960) 1. Introduction After reading an article [1], in the May 1959 issue of the Bulletin of the AMS wherein Mssrs. Hansen and Taft describe another method of computing double-theodolite runs, it was realized that a method developed at the AEC installation at Yucca Flat, Nevada is even more efficient than any previous method and could be of great value in speed and saving of man-hours to those wind- research installations that use the double-theodo- lite method. Further, it does not need special plotting boards but utilizes the standard Weather Bureau equipment with a few ingenious adaptations. FIG. 1. Winds-aloft plotting board, showing position of three distance scales. (360-deg intervals not indicated.) 2. Materials needed (1) Winds-aloft plotting board (fig. 1). A 3-ft-square board with distance scales running from the center to the bottom, with a movable circular piece of plastic attached to the center of the board, whose outer edge is marked off in 360-deg intervals. (2) Circular protractor (fig. 2). A 10-inch- square piece of clear plastic divided into 360-deg intervals. (3) Appropriately marked scale in the shape of an "L" (fig. 3). (4) Scotch tape, rubber band and short piece of string. (5) Winds-aloft graphing board (fig. 4). A rectangular board 2 X 2% ft marked with height FIG. 2. Clear plastic protractor, 10 inches square. -
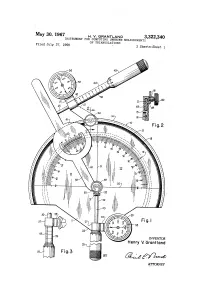
INVENTOR Henry V
May 30, 1967 H. W. GRANTAND 3,322,340 INSTRUMENT FOR COMPUTING UNKNOWN MEASUREMENTS Filed July 27, 1966 OF TRIANGULATIONS 3 Sheets-Sheet l 2 2 Z INVENTOR Henry V. Grant land ATTORNEY May 30, 1967 H. v. GRANTLAND 3,322,340 INSTRUMENT FOR cóMPUN UNKNOWN MEASUREMENTS OF TRIANGULATIONS Filed July 27, 1966 3 Sheets-Sheet a READS 30 FROM Vert CA READS 250 Fig. IO INVENTOR Henry V. Grontlond C = C, x C ose can C C - 250 x 2. OOO ... a = 5oo' au BY (2-4-6%2-2 Fig. ATTORNEY May 30, 1967 H. V. GRANTLAND 3,322,340 . INSTRUMENT FOR COMPUT ING UNKNOWN MEASUREMENTS OF TRIANGULATIONS Filed July 27, 1966 5. Sheets-Sheet : 3 READS 6O FROM VERTCA b = c x Cot angent C b = 250 x 1.732O b = 433' As 58-24S& 66 Fig. 16 5759-22 2S 2222 INVENTOR Henry V. Grant and Fig.19 BY (2-4%. ATTORNEY 3,322,340 United States Patent Office Patented May 30, 1967 1. 2 3,322,340 line 2-2 of FIGURE 1, illustrating the movable asso INSTRUMENT FOR COMPUTING UNKNOWN ciation between the upper micrometer and the protractor MEASUREMENTS OF TRANGULATIONS plate. Henry V. Grantland, Rte. 3, Box 105, FIGURE 3 is a fragmentary elevational illustration of Arlington, Tex. 76010 the lower micrometer barrel and the vernier dial mount Filed July 27, 1966, Ser. No. 568,250 ing attached thereto. 4 Claims. (C. 235-61) FIGURE 4 is a side elevational view of the invention. This invention relates to calculating devices, and it has FIGURE 5 is a fragmentary sectional view showing the particular reference to an instrument for computing un pivotal and slidable connection between the lower mi known measurements of either side of a triangle where O crometer and the angle scale, and between these elements the measurements and angulation of an acute angle and and the protractor plate. -

Rotating Vise Base P/N 3570
WEAR YOUR SAFETY GLASSES FORESIGHT IS BETTER THAN NO SIGHT READ INSTRUCTIONS BEFORE OPERATING Rotating Vise Base P/N 3570 Purpose of the Rotating Vise Base you will first have to indicate in the spindle in relation to The rotating vise base eliminates clamping and unclamping the center of the rotary base with the vise removed. Install the vise to produce angles. Once mounted square to the table, an indicator in the spindle and offset it to sweep the two the rotating base allows the vise to be positioned using the sides of the rotating base where the witness marks are laser engraved protractor scale as a guide for setting the (See Figure 1). Rotate the spindle and move the vise base angle. In addition, by loosening the clamping screws, the until you get zero deflection in the needle as you measure vise can be slid forward or backward in the mounting base both sides. Once centered, install a pointer in the spindle. as another way to change the position of the part. Mount your vise in the base and place the part in the vise jaws. Move the part sideways in the jaws to locate the left/ Using the Rotating Vise Base right centerline marked on your part with the pointer. Then The vise base is clamped to the mill table in the same move your vise forward or back until the pointer aligns manner as all Sherline accessories using the T-nuts in the with the other axis centerline. Now you can advance your table slots. If a high degree of accuracy is desired, the base handwheel the amount of the desired radius of your pattern should be squared up to the table by “indicating in” with to achieve the proper offset. -

Restoration of a 17Th Century Alta-Azimuth Theodolite FIGURE 1
Restoration of a 17th Century Alta-Azimuth Theodolite By Jeffrey Lock About 12 years ago I was fortunate to obtain a 17th century English theodolite complete with its original, octagonal box and Jacob’s staff adaptor (FIG. 1). At the time of acquisition I was not familiar with the names engraved on the arms of the azimuth plate. Once I began to research the names, I realized this was an important historical, as well as scientific, instrument. FIGURE 1 The maker’s name, Joseph Hone (FIG. 2) was mentioned in Gloria Clifton’s book1 and J. R. Millburn’s article, Some English Military Instrument Makers of the 17th Century.2 In Millburn’s article he noted, “The principal supplier of ‘mathematical’ instruments to the Government during the reigns of James II, William & Mary, and William III, seems to have been the relatively unknown Joseph Hone.” He continues, “No surviving signed examples of his products have been reported, so his abilities as an instrument maker - if indeed he was an actual maker – cannot be assessed.” Gloria Clifton confirmed that John Rowley began his apprenticeship to Hone on 23 November 1682. Rowley became an important instrument maker and also a supplier of instruments to the Board of Ordnance, as well as Christ’s Hospital, as was Joseph Hone. The signed theodolite serves as proof that Hone was a maker of exceptional quality, evidenced not only by the superior workmanship of the instrument but additionally by the very accurately engraved divisions on the theodolite’s azimuth circle. FIGURE 2 Research of the owner’s name, Sir John Colleton (FIG. -

CHAPTER 6 Technical Drawing Tools Based on Technical Graphics Communication by Bertoline, Et Al Dr Simin Nasseri, Southern Polytechnic State University
CHAPTER 6 Technical Drawing Tools Based on Technical Graphics Communication by Bertoline, et al Dr Simin Nasseri, Southern Polytechnic State University OBJECTIVES After completing this chapter, you will be able to: 1. Identify the important parts of a CAD system used to create technical drawings. 2. Define the important terms related to CAD systems. 3. Identify the important traditional tools used to create technical drawings. 4. Define the important terms related to traditional tools. 5. Use traditional tools and CAD to draw lines, circles, arcs, and curves. 6. Use scales, dividers, and CAD to measure and scale drawings. 7. Identify standard metric, U.S., and architectural drawing sheet sizes. 8. Identify standard pencil grades, and identify those most commonly used for technical drawings. 9. Identify the types and thicknesses of the various lines in the alphabet of lines. 10. Use traditional tools and CAD to erase parts of a drawing. INTRODUCTION Just as the graphics language has evolved over the years into a sophisticated set of standards and conventions, so have the tools used to graphically communicate technical ideas. Tools are used to produce three basic types of drawings: freehand sketches, instrument drawings, and computer drawings and models. 6.1 TECHNICAL DRAWING TOOLS Computer-aided design/drafting (CAD) is computer software and related computer hardware that supplements or replaces traditional hand tools in creating models and technical drawings. Engineers use computer-aided design/computer-aided manufacturing (CAD/CAM) in the design and production processes. 6.2 COMPUTER-AIDED DRAWING TOOLS A CAD system consists of hardware devices used in combination with specific software. -

ر ا تاذ ا Vernier Bevel Protractor
Basic Workshop أت ارش 1 Dr. Abdel-Wahab El-Morsy Faculty of Engineering - Rabigh Measuring Instruments Measuring Instruments Indirect measuring Direct measuring devices devices Caliper Vernier Caliper Radius Gauge Micrometer Plug Gauge Vernier Bevel Protractor Dr. Abdel-Wahab El-Morsy Faculty of Engineering - Rabigh Direct Measuring De ices ا-,+ ذات ار() Vernier Caliper A measuring tool for taking inside and outside measurements Measuring Range 0 –100, 0 –125, 0 –150,0 –200, ….. .ensiti ity 0.1, 0.05, 0.02 mm Dr. Abdel-Wahab El-Morsy Faculty of Engineering - Rabigh Direct Measuring De ices 11 2utside 3aws : used to measure external length 21 Inside 3aws : used to measure internal length 31 Depth probe : used to measure depth 81 Main scale (cm) 51 Main scale (inch) 61 Vernier (cm) 71 Vernier (inch) 81 Retainer : used to block/release movable part Dr. Abdel-Wahab El-Morsy Faculty of Engineering - Rabigh Direct Measuring De ices Reading the Vernier .cale Dr. Abdel-Wahab El-Morsy Faculty of Engineering - Rabigh Direct Measuring De ices Reading the Vernier .cale Dr. Abdel-Wahab El-Morsy Faculty of Engineering - Rabigh Direct Measuring De ices Reading the Vernier .cale Dr. Abdel-Wahab El-Morsy Faculty of Engineering - Rabigh Direct Measuring De ices Reading the Vernier .cale Dr. Abdel-Wahab El-Morsy Faculty of Engineering - Rabigh Direct Measuring De ices Vernier inside Vernier gauge Depth gauge Vernier outside • Range 0 – 100, 0 – 125, 0 - 200 mm gauge • .ensitivity 0.1, 0.05, 0.02 mm Vernier long outside gauge Dr. Abdel-Wahab El-Morsy Faculty of Engineering - Rabigh Direct Measuring De ices Vernier Beight gauge • Range 0 – 300, 0 – 500 mm • .ensitivity 0.1, 0.05, 0.02 mm Dr.