Influence of Cheese Making Technologies on Plasmin And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

In Escherichia Coli (Synthetic Oligonucleotide/Gene Expression/Industrial Enzyme) J
Proc. Nati Acad. Sci. USA Vol. 80, pp. 3671-3675, June 1983 Biochemistry Synthesis of calf prochymosin (prorennin) in Escherichia coli (synthetic oligonucleotide/gene expression/industrial enzyme) J. S. EMTAGE*, S. ANGALt, M. T. DOELt, T. J. R. HARRISt, B. JENKINS*, G. LILLEYt, AND P. A. LOWEt Departments of *Molecular Genetics, tMolecular Biology, and tFermentation Development, Celltech Limited, 250 Bath Road, Slough SL1 4DY, Berkshire, United Kingdom Communicated by Sydney Brenner, March 23, 1983 ABSTRACT A gene for calf prochymosin (prorennin) has been maturation conditions and remained insoluble on neutraliza- reconstructed from chemically synthesized oligodeoxyribonucleo- tion, it was possible that purification as well as activation could tides and cloned DNA copies of preprochymosin mRNA. This gene be achieved. has been inserted into a bacterial expression plasmid containing We describe here the construction of E. coli plasmids de- the Escherichia coli tryptophan promoter and a bacterial ribo- signed to express the prochymosin gene from the trp promoter some binding site. Induction oftranscription from the tryptophan and the isolation and conversion of this prochymosin to en- promoter results in prochymosin synthesis at a level of up to 5% active of total protein. The enzyme has been purified from bacteria by zymatically chymosin. extraction with urea and chromatography on DEAE-celiulose and MATERIALS AND METHODS converted to enzymatically active chymosin by acidification and neutralization. Bacterially produced chymosin is as effective in Materials. DNase I, pepstatin A, and phenylmethylsulfonyl clotting milk as the natural enzyme isolated from calf stomach. fluoride were obtained from Sigma. Calf prochymosin (Mr 40,431) and chymosin (Mr 35,612) were purified from stomachs Chymosin (rennin) is an aspartyl proteinase found in the fourth from 1-day-old calves (1). -

Cloning and in Vitro-Transcription of Chymosin Gene in E. Coli
The Open Nutraceuticals Journal, 2010, 3, 63-68 63 Open Access Cloning and In Vitro-Transcription of Chymosin Gene in E. coli S.A. El-Sohaimy1 Elsayed. E, Hafez2,* and M.A. El-Saadani3 1Food Science and Technology Department, Arid land Research Institute, Alexandria, Egypt 2Plant Molecular Pathology Department, Arid land Research Institute, Alexandria, Egypt 3Mubarak City for Scientific Research and Technology Applications, Alexandria, Egypt Abstract: Chymosin, commonly known as rennin, is the main milk-coagulating enzyme available in rennet. RNA was extracted from the abomasum of a suckling calf water buffalo and was subjected to RT-PCR using degenerate primers to amplify 850bp of the chymosin gene. The sequence was aligned with 19 different mammals' chymosin genes. The sequence revealed that there is a similarity to them ranging from 64% to 98%. The purified recombinant proteins were obtained from the transformed E. coli and yeast. The clotting activity of both of the resulting proteins was examined compared to the commercial peers. It was noticed that the concentration of the purified protein ranged from 15,000 to 40,000 MCU. Therefore, the activity of the obtained proteins was the same and it was 105% when compared to the commercial peer. Having examined the cytotoxicity of the purified proteins, the results revealed no toxicity. We can conclude that the obtained recombinant protein is more active and safe even when expressed in bacteria rather than yeast. Keywords: Chymosin, Milk Clotting, Recombinant E. coli and Recombinant Protein. INTRODUCTION Many microorganisms are known to produce rennet-like proteinases which can replace the calf rennet. -

Progress in the Field of Aspartic Proteinases in Cheese Manufacturing
Progress in the field of aspartic proteinases in cheese manufacturing: structures, functions, catalytic mechanism, inhibition, and engineering Sirma Yegin, Peter Dekker To cite this version: Sirma Yegin, Peter Dekker. Progress in the field of aspartic proteinases in cheese manufacturing: structures, functions, catalytic mechanism, inhibition, and engineering. Dairy Science & Technology, EDP sciences/Springer, 2013, 93 (6), pp.565-594. 10.1007/s13594-013-0137-2. hal-01201447 HAL Id: hal-01201447 https://hal.archives-ouvertes.fr/hal-01201447 Submitted on 17 Sep 2015 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Dairy Sci. & Technol. (2013) 93:565–594 DOI 10.1007/s13594-013-0137-2 REVIEW PAPER Progress in the field of aspartic proteinases in cheese manufacturing: structures, functions, catalytic mechanism, inhibition, and engineering Sirma Yegin & Peter Dekker Received: 25 February 2013 /Revised: 16 May 2013 /Accepted: 21 May 2013 / Published online: 27 June 2013 # INRA and Springer-Verlag France 2013 Abstract Aspartic proteinases are an important class of proteinases which are widely used as milk-coagulating agents in industrial cheese production. They are available from a wide range of sources including mammals, plants, and microorganisms. -

Serine Proteases with Altered Sensitivity to Activity-Modulating
(19) & (11) EP 2 045 321 A2 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 08.04.2009 Bulletin 2009/15 C12N 9/00 (2006.01) C12N 15/00 (2006.01) C12Q 1/37 (2006.01) (21) Application number: 09150549.5 (22) Date of filing: 26.05.2006 (84) Designated Contracting States: • Haupts, Ulrich AT BE BG CH CY CZ DE DK EE ES FI FR GB GR 51519 Odenthal (DE) HU IE IS IT LI LT LU LV MC NL PL PT RO SE SI • Coco, Wayne SK TR 50737 Köln (DE) •Tebbe, Jan (30) Priority: 27.05.2005 EP 05104543 50733 Köln (DE) • Votsmeier, Christian (62) Document number(s) of the earlier application(s) in 50259 Pulheim (DE) accordance with Art. 76 EPC: • Scheidig, Andreas 06763303.2 / 1 883 696 50823 Köln (DE) (71) Applicant: Direvo Biotech AG (74) Representative: von Kreisler Selting Werner 50829 Köln (DE) Patentanwälte P.O. Box 10 22 41 (72) Inventors: 50462 Köln (DE) • Koltermann, André 82057 Icking (DE) Remarks: • Kettling, Ulrich This application was filed on 14-01-2009 as a 81477 München (DE) divisional application to the application mentioned under INID code 62. (54) Serine proteases with altered sensitivity to activity-modulating substances (57) The present invention provides variants of ser- screening of the library in the presence of one or several ine proteases of the S1 class with altered sensitivity to activity-modulating substances, selection of variants with one or more activity-modulating substances. A method altered sensitivity to one or several activity-modulating for the generation of such proteases is disclosed, com- substances and isolation of those polynucleotide se- prising the provision of a protease library encoding poly- quences that encode for the selected variants. -

Camel and Bovine Chymosin: the Relationship Crystallography Between Their Structures and Cheese-Making ISSN 0907-4449 Properties
research papers Acta Crystallographica Section D Biological Camel and bovine chymosin: the relationship Crystallography between their structures and cheese-making ISSN 0907-4449 properties Jesper Langholm Jensen,a,b Bovine and camel chymosin are aspartic peptidases that are Received 19 November 2012 Anne Mølgaard,a Jens-Christian used industrially in cheese production. They cleave the Accepted 31 January 2013 Navarro Poulsen,a Phe105-Met106 bond of the milk protein -casein, releasing b Marianne Kirsten Harboe, its predominantly negatively charged C-terminus, which leads PDB References: bovine Jens Bæk Simonsen,c‡ to the separation of the milk into curds and whey. Despite chymosin, 4aa8; camel chymosin, 4aa9 Andrea Maria Lorentzen,d Karin having 85% sequence identity, camel chymosin shows a 70% higher milk-clotting activity than bovine chymosin towards Hjernø,d Johannes M. van den bovine milk. The activities, structures, thermal stabilities and Brink,b Karsten Bruun Qvistb and a glycosylation patterns of bovine and camel chymosin obtained Sine Larsen * by fermentation in Aspergillus niger have been examined. Different variants of the enzymes were isolated by hydro- aDepartment of Chemistry, University of phobic interaction chromatography and showed variations Copenhagen, Denmark, bChr. Hansen A/S, in their glycosylation, N-terminal sequences and activities. Bøge Alle´ 10-12, DK-2970 Hørsholm, Glycosylation at Asn291 and the loss of the first three residues c Denmark, Nanobioscience, Department of of camel chymosin significantly decreased its activity. Thermal Basic Sciences and Environment, University of Copenhagen, Denmark, and dInstitute of differential scanning calorimetry revealed a slightly higher Biochemistry and Molecular Biology, thermal stability of camel chymosin compared with bovine University of Southern Denmark, Denmark chymosin. -

Penicillopepsin-JT2, a Recombinant Enzyme from Penicillium Janthinellum and the Contribution of a Hydrogen Bond in Subsite S3 to Kcat
Protein Science ~2000!, 9:991–1001. Cambridge University Press. Printed in the USA. Copyright © 2000 The Protein Society Penicillopepsin-JT2, a recombinant enzyme from Penicillium janthinellum and the contribution of a hydrogen bond in subsite S3 to kcat QING-NA CAO,1,3 MARLENE STUBBS,1 KENNY Q.P. NGO,1 MICHAEL WARD,2 ANNIE CUNNINGHAM,1 EMIL F. PAI,1 GUANG-CHOU TU,1,3 and THEO HOFMANN1 1 Department of Biochemistry, University of Toronto, Toronto, Ontario M5S 1A8, Canada 2 Genencor International, Inc., 925 Page Mill Road, Palo Alto, California 94304-1013 ~Received August 30, 1999; Final Revision February 7, 2000; Accepted March 10, 2000! Abstract The nucleotide sequence of the gene ~ pepA! of a zymogen of an aspartic proteinase from Penicillium janthinellum with a 71% identity in the deduced amino acid sequence to penicillopepsin ~which we propose to call penicillopepsin-JT1! has been determined. The gene consists of 60 codons for a putative leader sequence of 20 amino acid residues, a sequence of about 150 nucleotides that probably codes for an activation peptide and a sequence with two introns that codes for the active aspartic proteinase. This gene, inserted into the expression vector pGPT-pyrG1, was expressed in an aspartic proteinase-free strain of Aspergillus niger var. awamori in high yield as a glycosylated form of the active enzyme that we call penicillopepsin-JT2. After removal of the carbohydrate component with endoglycosidase H, its relative molecular mass is between 33,700 and 34,000. Its kinetic properties, especially the rate-enhancing effects of the presence of alanine residues in positions P3 and P29 of substrates, are similar to those of penicillopepsin-JT1, endothia- pepsin, rhizopuspepsin, and pig pepsin. -

Hydrolysis of -Lactalbumin by Chymosin and Pepsin. Effect Of
Hydrolysis of α-lactalbumin by chymosin and pepsin. Effect of conformation and pH G. Miranda, G. Hazé, Non Renseigné To cite this version: G. Miranda, G. Hazé, Non Renseigné. Hydrolysis of α-lactalbumin by chymosin and pepsin. Effect of conformation and pH. Le Lait, INRA Editions, 1989, 69 (6), pp.451-459. hal-00929176 HAL Id: hal-00929176 https://hal.archives-ouvertes.fr/hal-00929176 Submitted on 1 Jan 1989 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Lait (1989) 69. 451-459 451 © Elsevier/INRA Original article Hydrolysis of œ-lactalburnin by chymosin and pepsin. Effect of conformation and pH G. Miranda, G. Hazé, P.Scanff and J.P.Pélissier INRA, station de recherches laitières, 78350 Jouy-en-Josas, France (received 21 March 1989. accepted 26 June 1989) Summary - The correlation between change of conformation of a-Iactalbumin and its degradation by gastric enzymes was verified. With citrate buffer (0.1 M), the modification of a-Iactalbumin confor- mation occurred when the pH value was below pH 4.0. This conformational change was influenced by buffer composition and ionic strength. However, the presence of EDTA in the butter did not modi- fy the pH value at which the change of conformation of the protein occurred. -

In Silico Drug Activity Prediction of Chemical Components of Acalypha Indica Dr
International Journal of Scientific Engineering and Applied Science (IJSEAS) – Volume-2, Issue-6,June 2016 ISSN: 2395-3470 www.ijseas.com In Silico drug activity prediction of chemical components of Acalypha Indica Dr. (Mrs.)S.Shanthi M.Sc.,M.Phil.,Ph.D. Associate professor Department of chemistry,SFR college,Sivakasi. S.Sri Nisha Tharani M.Phil Chemistry ,Department of Chemistry,SFR College,Sivakasi,Tamilnadu,India. ABSTRACT Acalypha indica is a common annual Acalypha indica distributed in the herb, found mostly in the backyards of southern part of India, particularly in houses and waste places throughout the Tamilnadu has potential medicinal plains of India. Plants are used as emetic, properties and used as diuretic, anthelmintic expectorant, laxative, diuretic , bronchitis, and for respiratory problems such as pneumonia, asthma and pulmonary [1] bronchitis, asthma and pneumonia. tuberculosisP .P Leaves are laxative and Acalypha indica plant contains alkaloids, antiparasiticide; ground with common salt or tannins, steroids, saponins, terpenoids, quicklime or lime juice applied externally in flavanoids, cardiac glycosides and phenolic scabies. Leaf paste with lime juice is compounds. Some chemical components prescribed for ringworm; leaf juice is emetic were selected to theoretically evaluate their for children. A decoction of the leaves is drug likeness score using some drug given in earache. Powder of the dry leaves is designing softwares.. Their molecular given to children to expell worms; also properties were calculated using the given in the form of decoction with little software Molinspiration., Prediction of garlic. In homoepathy, the plant is used in biological activities and pharmacological severe cough associated with bleeding from activities were done using PASS online. -

Handbook of Proteolytic Enzymes Second Edition Volume 1 Aspartic and Metallo Peptidases
Handbook of Proteolytic Enzymes Second Edition Volume 1 Aspartic and Metallo Peptidases Alan J. Barrett Neil D. Rawlings J. Fred Woessner Editor biographies xxi Contributors xxiii Preface xxxi Introduction ' Abbreviations xxxvii ASPARTIC PEPTIDASES Introduction 1 Aspartic peptidases and their clans 3 2 Catalytic pathway of aspartic peptidases 12 Clan AA Family Al 3 Pepsin A 19 4 Pepsin B 28 5 Chymosin 29 6 Cathepsin E 33 7 Gastricsin 38 8 Cathepsin D 43 9 Napsin A 52 10 Renin 54 11 Mouse submandibular renin 62 12 Memapsin 1 64 13 Memapsin 2 66 14 Plasmepsins 70 15 Plasmepsin II 73 16 Tick heme-binding aspartic proteinase 76 17 Phytepsin 77 18 Nepenthesin 85 19 Saccharopepsin 87 20 Neurosporapepsin 90 21 Acrocylindropepsin 9 1 22 Aspergillopepsin I 92 23 Penicillopepsin 99 24 Endothiapepsin 104 25 Rhizopuspepsin 108 26 Mucorpepsin 11 1 27 Polyporopepsin 113 28 Candidapepsin 115 29 Candiparapsin 120 30 Canditropsin 123 31 Syncephapepsin 125 32 Barrierpepsin 126 33 Yapsin 1 128 34 Yapsin 2 132 35 Yapsin A 133 36 Pregnancy-associated glycoproteins 135 37 Pepsin F 137 38 Rhodotorulapepsin 139 39 Cladosporopepsin 140 40 Pycnoporopepsin 141 Family A2 and others 41 Human immunodeficiency virus 1 retropepsin 144 42 Human immunodeficiency virus 2 retropepsin 154 43 Simian immunodeficiency virus retropepsin 158 44 Equine infectious anemia virus retropepsin 160 45 Rous sarcoma virus retropepsin and avian myeloblastosis virus retropepsin 163 46 Human T-cell leukemia virus type I (HTLV-I) retropepsin 166 47 Bovine leukemia virus retropepsin 169 48 -

Molecular Cloning and Characterization of Procirsin, an Active Aspartic Protease Precursor from Cirsium Vulgare (Asteraceae)
Phytochemistry 81 (2012) 7–18 Contents lists available at SciVerse ScienceDirect Phytochemistry journal homepage: www.elsevier.com/locate/phytochem Molecular cloning and characterization of procirsin, an active aspartic protease precursor from Cirsium vulgare (Asteraceae) Daniela Lufrano a, Rosário Faro b, Pedro Castanheira c, Gustavo Parisi d, Paula Veríssimo b,e, ⇑ Sandra Vairo-Cavalli a, Isaura Simões b,c, , Carlos Faro b,c,e a Laboratorio de Investigación de Proteínas Vegetales (LIPROVE), Departamento de Ciencias Biológicas, Facultad de Ciencias Exactas, Universidad Nacional de La Plata, C.C. 711, 1900 La Plata, Argentina b CNC-Center for Neuroscience and Cell Biology, University of Coimbra, 3004-517 Coimbra, Portugal c Biocant, Biotechnology Innovation Center, 3060-197 Cantanhede, Portugal d Departamento de Ciencia y Tecnología, Universidad Nacional de Quilmes, Roque Sáenz Peña 352, Bernal, Buenos Aires, B1876BXD, Argentina e Department of Life Sciences, University of Coimbra, 3004-517 Coimbra, Portugal article info abstract Article history: Typical aspartic proteinases from plants of the Astereaceae family like cardosins and cyprosins are well- Received 16 February 2012 known milk-clotting enzymes. Their effectiveness in cheesemaking has encouraged several studies on Received in revised form 23 May 2012 other Astereaceae plant species for identification of new vegetable rennets. Here we report on the clon- Available online 21 June 2012 ing, expression and characterization of a novel aspartic proteinase precursor from the flowers of Cirsium vulgare (Savi) Ten. The isolated cDNA encoded a protein product with 509 amino acids, termed cirsin, Keywords: with the characteristic primary structure organization of plant typical aspartic proteinases. The pro form Cirsium vulgare of cirsin was expressed in Escherichia coli and shown to be active without autocatalytically cleaving its Asteraceae pro domain. -

Supplementary Materials
Electronic Supplementary Material (ESI) for New Journal of Chemistry. This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2017 Supplementary Materials Dihydro-2H-thiopyran-3(4H)-one-1,1-dioxide - a versatile building block for the synthesis of new thiopyran-based heterocyclic systems Vitalii A. Palchykov,*a Roman M. Chabanenko,a Valeriy V. Konshin,b Victor V. Dotsenko,b,c Sergey G. Krivokolysko,b Elena A. Chigorina,d Yuriy I. Horak,e Roman Z. Lytvyn,e Andriy A. Vakhula,e Mykola D. Obushake and Alexander V. Mazepaf aDepartment of Organic Chemistry, Oles Honchar Dnipro National University, 72 Gagarina Av, 49010 Dnipro, Ukraine bDepartment of Chemistry & High Technologies, Kuban State University, 149 Stavropolskaya St, 350040 Krasnodar, Russian Federation сDepartment of Chemistry, North Caucasus Federal University, 1a Pushkin St, 355009 Stavropol, Russian Federation dFederal State Unitary Enterprise «State Scientific Research Institute of Chemical Reagents and High Purity Chemical Substances» (FSUE «IREA»), 3 Bogorodsky val St, 107076 Moscow, Russian Federation eDepartment of Organic Chemistry, Ivan Franko National University of Lviv, 6 Kyryla i Mefodiya St, 79005 Lviv, Ukraine fA. V. Bogatsky Physico-Chemical Institute, National Academy of Sciences of Ukraine, 86 Lustdorfskaya Rd, 65080 Odessa, Ukraine *E-mail: [email protected] 1 CONTENTS: Page: 1 H and 13C NMR spectra of compound 2a (400/100 MHz, DMSO-d6)................................ 4 IR spectrum of compound 2a ............................................................................................. 4 Mass spectrum (EI) of compound 2a ................................................................................. 5 1 H-1H COSY spectrum of compound 2a (400 MHz, DMSO-d6) .......................................... 5 NOESY spectrum of compound 2a (400 MHz, DMSO-d6) ................................................. 6 1 H-13C HSQC spectrum of compound 2a (400/100 MHz, DMSO-d6) ................................ -
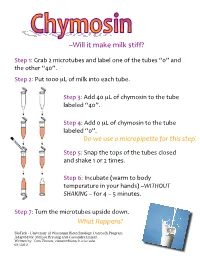
Chymosin: a Puzzle in Designing Experiments to Test Many Possible Explanations
LLM.55%."%-*?#%-.5?%2".66N% !"#$%&'%()*+%,%-./)0"1+#2%*34%5*+#5%03#%06%"7#%"1+#2%89:%*34% "7#%0"7#)%8;9:<% !"#$%,'%=1"%&999%µ>%06%-.5?%.3"0%#*/7%"1+#<% % !"#$%@'%A44%;9%µ>%06%/7B-02.3%"0%"7#%"1+#% 5*+#5#4%8;9:<% % !"#$%;'%A44%9%µ>%06%/7B-02.3%"0%"7#%"1+#% 5*+#5#4%89:<%%%%%%%%%%%%%%%%%%%%%%%%%% % -.'/0'120'3'4567.8580990':.7'9;52'2908< !"#$%C'%!3*$%"7#%"0$2%06%"7#%"1+#2%/502#4% *34%27*?#%&%0)%,%".-#2<%% % !"#$%D'%E3/1+*"#%FG*)-%"0%+04B% "#-$#)*"1)#%.3%B01)%7*342H%I!"#$%&#''''''''' ($)*"+,'I%60)%;%I%C%-.31"#2<%% % !"#$%J'%K1)3%"7#%-./)0"1+#2%1$2.4#%40G3<%% !;39'$3880=2< BioTrek - University of Wisconsin Biotechnology Outreach Program Adapted by: Melissa Breunig and Cassandra Immel Written by: Tom Zinnen, [email protected] 03/2010 Chymosin: A Puzzle in Designing Experiments to Test Many Possible Explanations • Chymosin is the protein that is the active enzyme in rennet used in making cheese. • Chymosin makes milk stiff, and then the cheesemaker separates the stiff milk into curds and whey. • There are at least two sources of chymosin. First, chymosin can be purified from the stomachs of young calves. • Second, chymosin can be made in bacteria that through gene splicing technology contain a copy of the cow gene for chymosin. • Chymosin is also the first product of gene splicing (recombinant DNA technology) in the US food supply. Recipe for Using Chymosin to Make Milk Stiff 1.Put 500 microliters (half a milliliter) of milk into a small plastic test tube with a lid (a microcentrifuge tube).